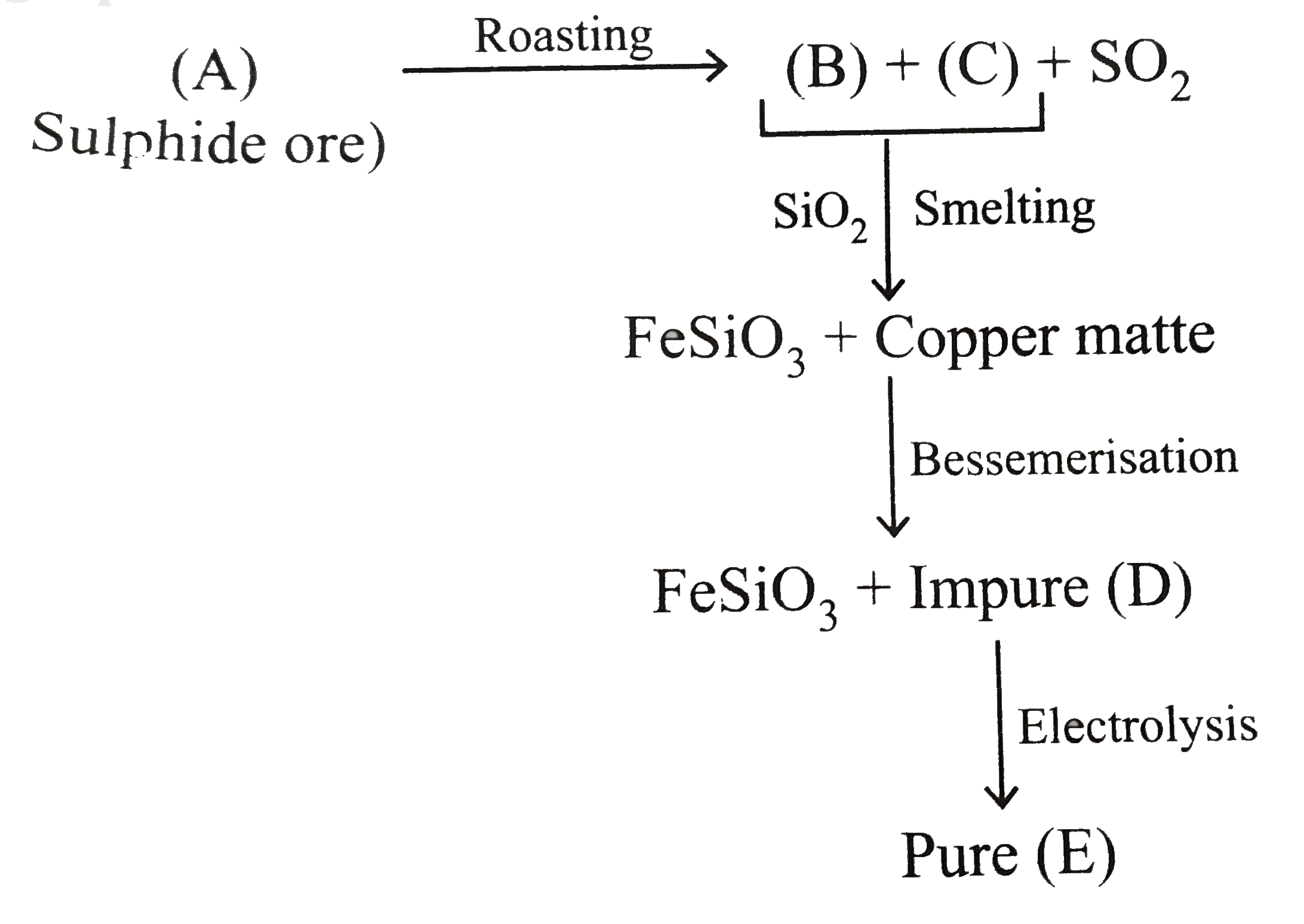A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Multiple Correct)|43 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Single Correcttype)|106 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exs 1.1 (True Orfalse Statement)|19 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archieves Subjective|35 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Linked Comprehension)|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS-Exercises (Linked Comprehension)
- B is :
Text Solution
|
- C is.
Text Solution
|
- E is.
Text Solution
|
- F is.
Text Solution
|
- underset(("Sulphide ore")) (A) + NaCN overset ("Air") hArr underset(("...
Text Solution
|
- underset(("Sulphide ore")) (A) + NaCN overset ("Air") hArr underset(("...
Text Solution
|
- underset(("Sulphide ore")) (A) + NaCN overset ("Air") hArr underset(("...
Text Solution
|
- underset(("Sulphide ore")) (A) + NaCN overset ("Air") hArr underset(("...
Text Solution
|
- Name the process (A)
Text Solution
|
- Name the process (B).
Text Solution
|
- Name the process ( C)
Text Solution
|
- Identify (A).
Text Solution
|
- Identify (B) and ( C) :
Text Solution
|
- Composition of copper matte is. (a)C u (2) S + F e S (small amount) ...
Text Solution
|
- Identify ( E).
Text Solution
|
- Pure ( E) is
Text Solution
|
- Chief ore of Zn is ZnS. The ore is concentrated by froth flotation pro...
Text Solution
|
- Chief ore of Zn is ZnS. The ore is concentrated by froth flotation pro...
Text Solution
|
- Chief ore of Zn is ZnS. The ore is concentrated by froth flotation pro...
Text Solution
|
- Chief ore of Zn is ZnS. The ore is concentrated by froth flotation pro...
Text Solution
|