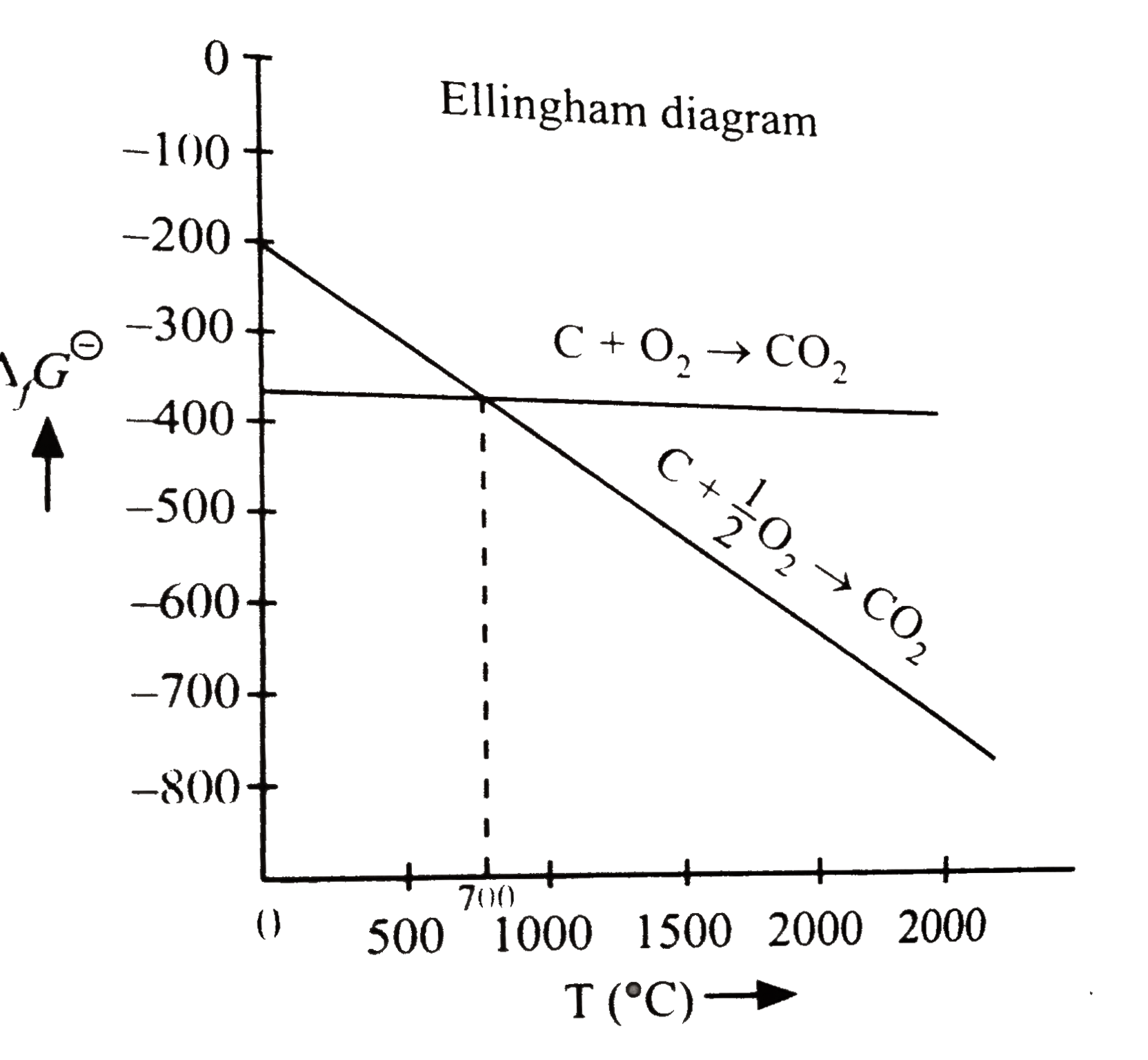
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Assertion-Reasoning)|18 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Integer)|27 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Multiple Correct)|43 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archieves Subjective|35 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Linked Comprehension)|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS-Exercise (Single Correcttype)
- During smelting, an additional is added which combines with impurites ...
Text Solution
|
- The process of isolation of metals by dissolving the ore in a suitable...
Text Solution
|
- Complexes formed in the following methods are : (1) Mond’s process ...
Text Solution
|
- Casseterite ore consists of magnetic impurity named as
Text Solution
|
- Find the formula of A ZnS + O(2) rarr (A) + SO(2).
Text Solution
|
- Four metals and their methods of refinement are given (i) Ni, Cu, Z...
Text Solution
|
- Which of the following statement is correct regarding Cu extraction ?
Text Solution
|
- Which of the following process is not involved in the extraction of Fe...
Text Solution
|
- Carbon reduction process is not commercially applicable for which of t...
Text Solution
|
- Which of the following metal can be reduced by carbon reduction as wel...
Text Solution
|
- Which of the following factors is of no significance for roasting sul...
Text Solution
|
- The method not used in metallurgy to refine impure metal is :
Text Solution
|
- The elements of group 16 are called as chalcogens. Give reason.
Text Solution
|
- The oxidation states of Cu and Fe in chalcopyrite are, respectively,
Text Solution
|
- Thermite reduction is not used for commercial extraction of the respec...
Text Solution
|
- Which of the following is incorrect on the basic of the above Ellingha...
Text Solution
|
- Copper can be extracted by hydrometallurgy but not zinc. Explain.
Text Solution
|
- Which of the following statement is correct ?
Text Solution
|
- Consider the following metallurgical processes : (1) Heating impure ...
Text Solution
|
- Consider the following statements : Roasting is carried out to : 1...
Text Solution
|