Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|7 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Application Exercises 2.1 (Subjective)|22 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|24 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY-Exercises Archives (Subjective)
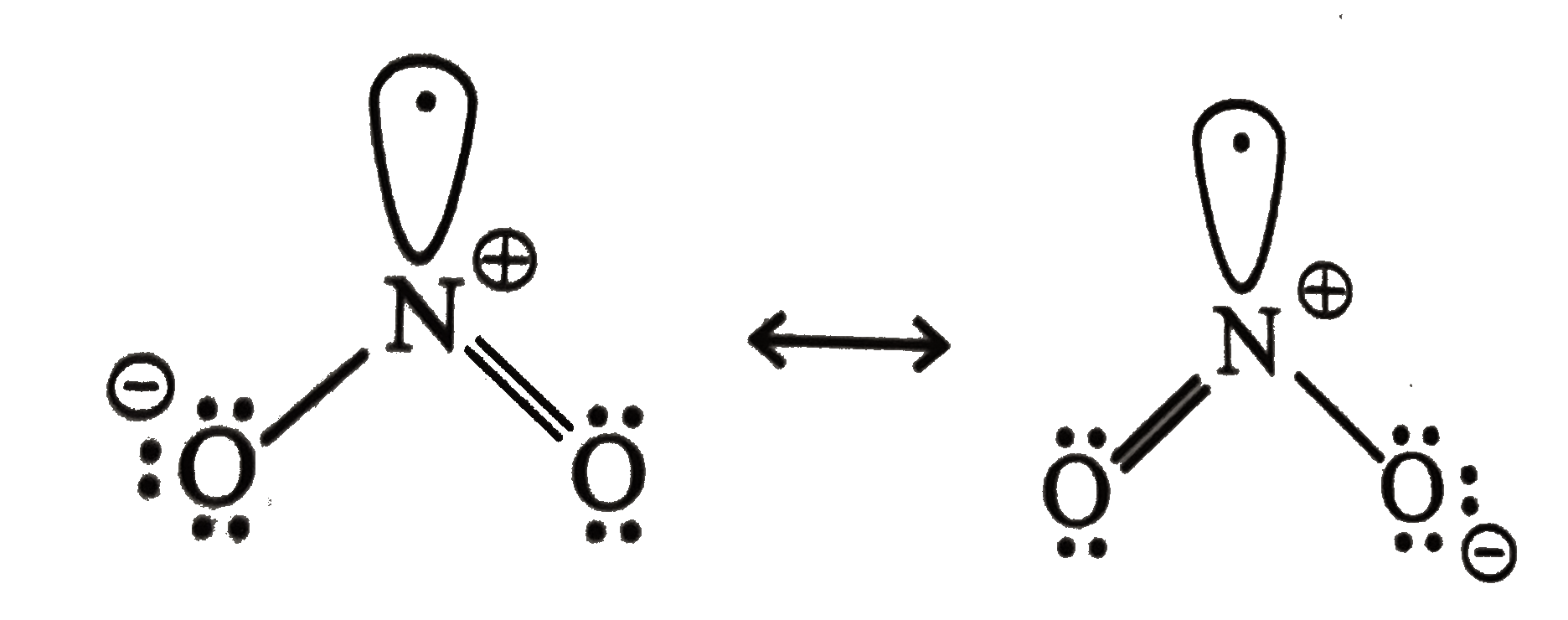
- Why does NO(2) dimerise ?
Text Solution
|
- Write the balanced equation involved in the preparation of (a) bleac...
Text Solution
|
- Explan the following : (i) Concentrated HNO3 turns yellow in sunlight...
Text Solution
|
- Give the structural formula for the following : (i) Phosphorous acid...
Text Solution
|
- Explain why 'orthophosphoric acid, H3 PO4, is tribasic but phosphorus ...
Text Solution
|
- Write the resonance structure of nitrous oxide.
Text Solution
|
- (i) Write the balanced equations for the reactions when ammonium sulph...
Text Solution
|
- Write balanced equations for the following : (i) Phosphorus is react...
Text Solution
|
- Explain the following in one or two sentances only : "Orthophosphor...
Text Solution
|
- Give balanced equations for the following : Phosphorous reacts with ...
Text Solution
|
- Write the balanced chemical equations when hypophosphorus acid is heat...
Text Solution
|
- Explain the following (i)H3 PO3 is a dibasic acid. (ii) Phosphine ...
Text Solution
|
- Write balanced equation for (i) The preparation of phosphine from Ca...
Text Solution
|
- Write two resonating structures of N(2)O that satify octet rule.
Text Solution
|
- Write balanced chemical equations for the following : (i) Sodium ni...
Text Solution
|
- The correct increasing order of extent of hydrolysis is :
Text Solution
|
- Give reason in one or two sentences. "Ammonium chloride is acidic in...
Text Solution
|
- Complete and balance the following chemical reactions : Red phosphorus...
Text Solution
|
- Identify the compounds A and B. PCl5 + SO2 rarr A + B .
Text Solution
|
- Complete and balance the following reactions. Ca5(PO4)3 F + H2 SO4 +...
Text Solution
|
- Account for the following : Mg3 N2 when reacted with water gives NH3...
Text Solution
|