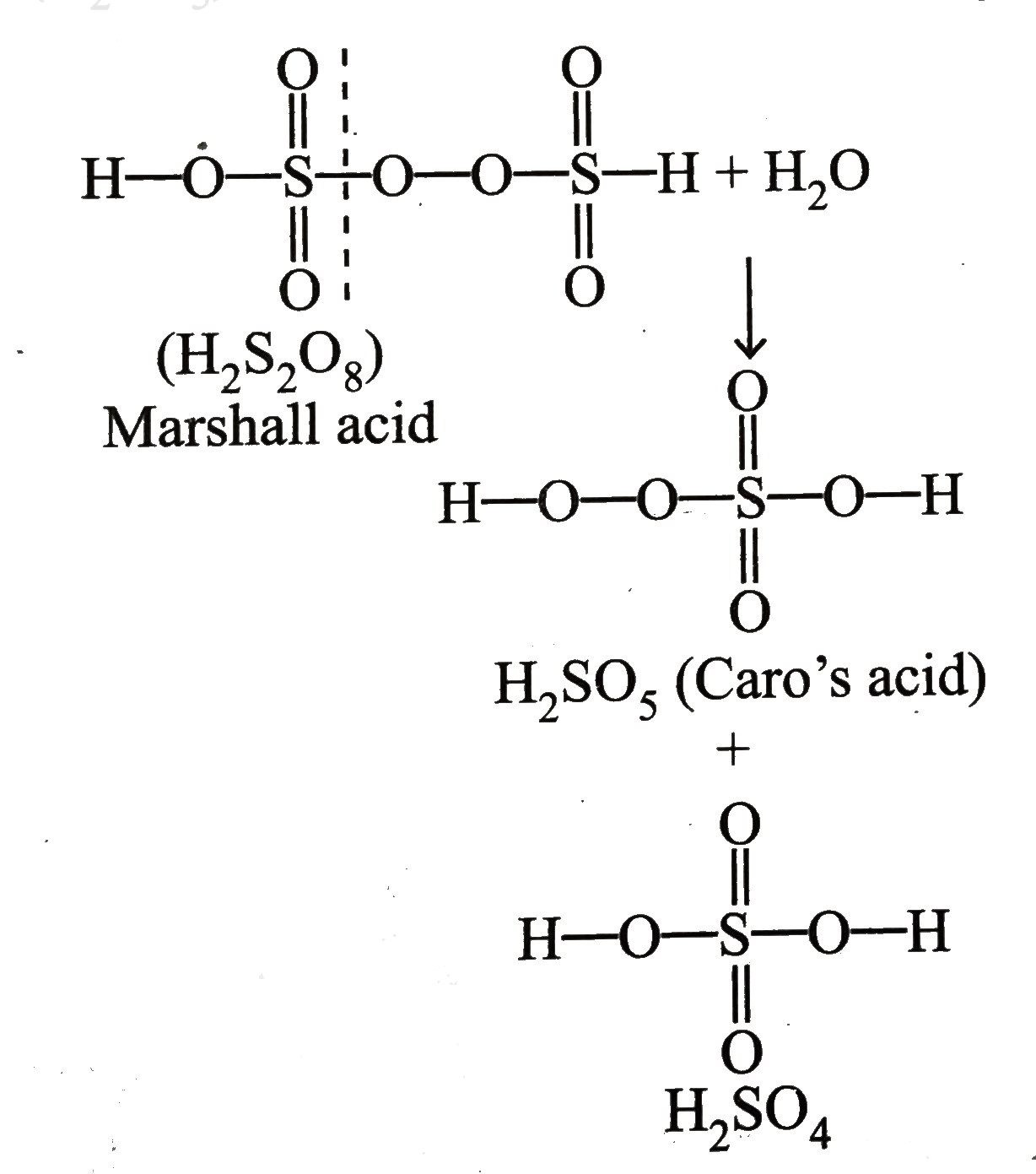
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Integer|4 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives(Integer)|1 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Multiple Correct|1 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|28 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise True/False (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY-Archives Single Correct
- The oxide that gives H(2)O(2) on treatment with a dilute sulfuric acid...
Text Solution
|
- There is no S-S bond in .
Text Solution
|
- The oxidation states of the most electronegative elements in the produ...
Text Solution
|
- The species that do not contain peroxide ions is
Text Solution
|
- Hydrolysis of one mole of peroxodisulphuric acid produces
Text Solution
|
- Which of the following oxides is neutral ?
Text Solution
|
- Which of the following has the highest boiling point ? H(2)O,H(2)S,H...
Text Solution
|
- The number of S -S bonds in sulphur trioxide trimer [S(3)O9] is
Text Solution
|
- Which of the following will not be oxidised by O(3) ?
Text Solution
|
- The species having pyramidal shape is :
Text Solution
|
- Which of the following does not give oxygen on heating?
Text Solution
|