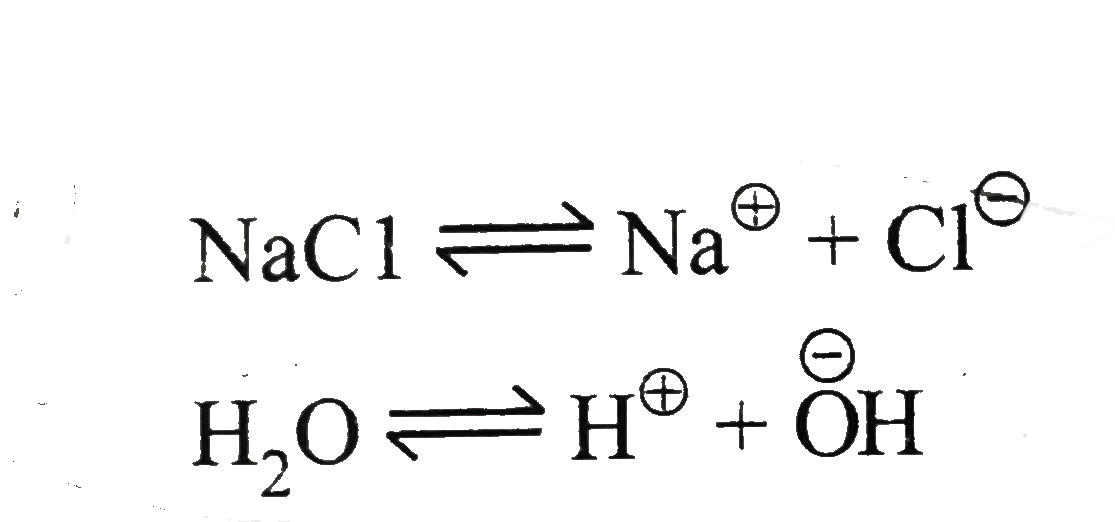Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exs 4.1 (Objective)|7 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Linked Comprehension)|26 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|7 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|10 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 (Objective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY-Exs 4.1 (Subjective)
- Bond dissociation energy of F(2) is less that of Cl(2) give reason.
Text Solution
|
- Why fluorine does not exhibit any positive oxidation state?
Text Solution
|
- The bleaching action of chlorine is permanent, whereas the bleaching a...
Text Solution
|
- Arrange HOCl, HClO(2), HClO(3) and HClO(4) in order of (i) acidic stre...
Text Solution
|
- Explain why the electrons gain enthalpy of fluorine is less negative t...
Text Solution
|
- Fluorine is more electronegative than iodine atom, yet HF has lower ac...
Text Solution
|
- What happen when Cl(2) is passed through a hot concentrated solution o...
Text Solution
|
- Why fluorine never acts as the centreal atom in polyatomic interhaloge...
Text Solution
|
- CIF3 exists but FCl3 does not exist. Why?
Text Solution
|
- Addition of Cl(2) turns it colourless. Why?
Text Solution
|
- Given relevent chemical equations for the preparation of a.HBr from ...
Text Solution
|
- A sodium salt (A) is heated with cone. Sulphuric acid. The evolved gas...
Text Solution
|
- A certain compound (X) shows the following reactions. (a) when KI is...
Text Solution
|
- A colourless inorganic compound (A) imparts a green colour to flame. I...
Text Solution
|
- Give balanced chemical reactions for the following: i. Sodium iodat...
Text Solution
|
- Pseudohalogens or halogenides are complex molecules which behaves like...
Text Solution
|