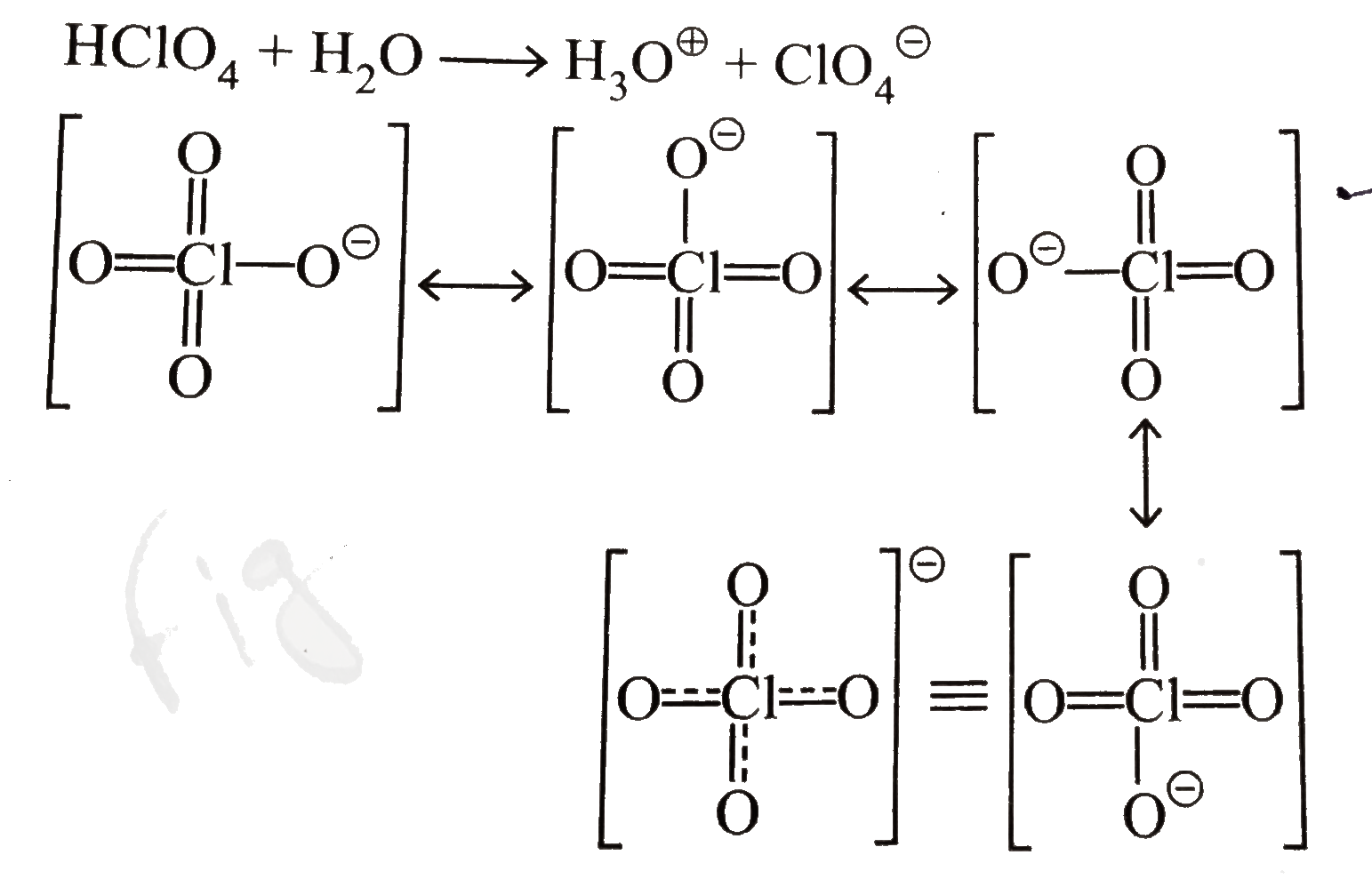
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Integer)|3 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Fill In The Blanks)|1 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Multiple Correct)|3 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|10 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 (Objective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY-Exercises Archives (Single Correct)
- HBr and HI can reduce sulphurie acid, HCI can reduced KMnO(4) and HF c...
Text Solution
|
- Chlorine acts as a bleaching agent only in the presence of
Text Solution
|
- Bromine can be liberated form potassium bromide solution by the action...
Text Solution
|
- The following acids have been arranged in order of decreasing acid str...
Text Solution
|
- Which of the following species is not a pseudo halide ?
Text Solution
|
- In compounds of type ECl(3), where E=B,P. As or Bi, the angles Cl-E-Cl...
Text Solution
|
- The correct order of acid strength is -
Text Solution
|
- The set with the correct order of acidity is
Text Solution
|
- The product of oxidation of I^(-) with MnO(4)^(-) in alkaline medium i...
Text Solution
|
- The correct statement for the molecule, Csl(3) is
Text Solution
|
- Among the following oxoacids, the correct decreasing order of acid str...
Text Solution
|
- Hydrogen peroxide in its reaction with KIO(4) and NH(4)OH respectively...
Text Solution
|