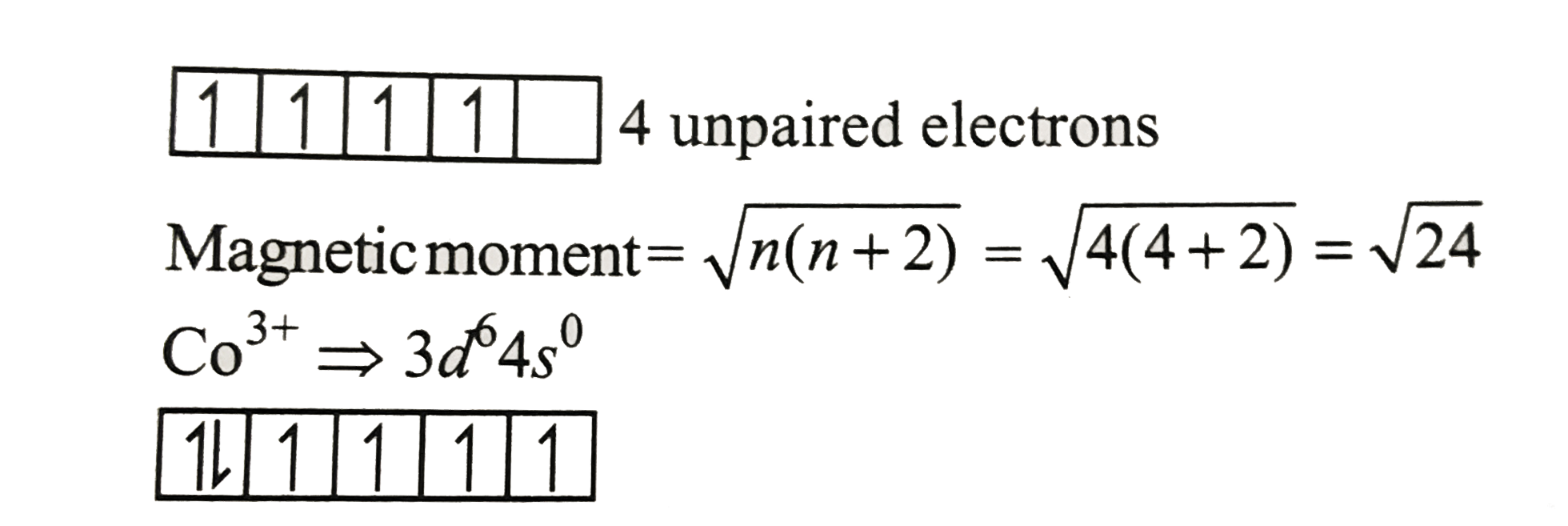Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct D-Block Elements (General Properties And Electronic Configuration)|15 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct D-Block Elements (Colour)|3 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|31 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|18 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archieves Subjective|35 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-D AND F BLOCK ELEMENTS-Exercises Multiple Correct
- d(x)2-(y)2 orbital is involved in which of the following hybridisation...
Text Solution
|
- Which of following is/are correctly matched ?
Text Solution
|
- Electrons are filled into atomic orbitals in the increasing order of o...
Text Solution
|
- Which of the following represents the incoorect order of the propertie...
Text Solution
|
- What will be the correct representation of quatum numbers f the last e...
Text Solution
|
- Which is true statement about KMnO4?
Text Solution
|
- Out of [Fe(CN)6]^(4-),[Ni(CN)4]^(2-), and [Ni(CO)4]: select the incorr...
Text Solution
|
- The ability of d-block elements to form complexes is due to:
Text Solution
|
- Which one of the following ionic species will impact colour to an aque...
Text Solution
|
- A transition element X has a configuration 3d^4 in its +3 oxidation st...
Text Solution
|
- The transition metals which do not form amalgams are
Text Solution
|
- The colour of the transition metal ions is due to
Text Solution
|
- Transition elements have greater tendency to form complexes because
Text Solution
|
- Which out of the following belong to 3d-series?
Text Solution
|
- The elements which exist in the liquid state at rooom temperature are.
Text Solution
|
- Which of the following statements (s) is (are) correct with reference ...
Text Solution
|
- Which of the following represents the correct order of the properties ...
Text Solution
|
- The correct statement for d-block element is
Text Solution
|
- The aqueous solution of the salt will be coloured in the case of
Text Solution
|
- Potassium manganate (K2MnO4) is formed when
Text Solution
|