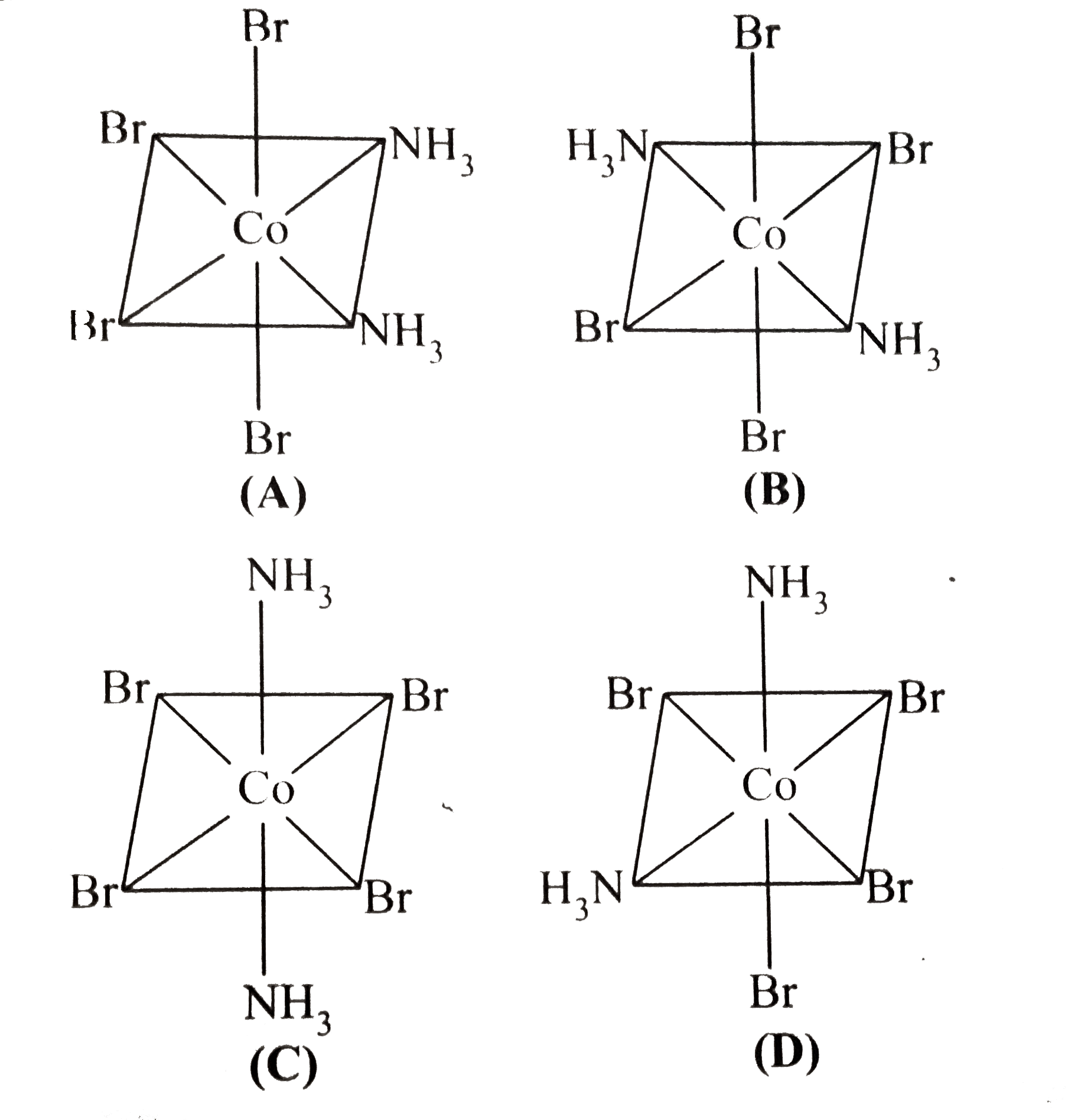
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct(Naming And Terminology)|9 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct(Isomerism )|10 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 7.2 Objective|8 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-COORDINATION COMPOUNDS-Exercises Linked Comprehension
- Consider to following isomers of [Co(NH(3))(2)Br(4)]^(Θ) and answer th...
Text Solution
|
- Consider to following isomers of [Co(NH(3))(2)Br(4)]^(Θ) and answer th...
Text Solution
|
- Consider to following isomers of [Co(NH(3))(2)Br(4)]^(Θ) and answer th...
Text Solution
|
- Consider to following isomers of [Co(NH(3))(2)Br(4)]^(Θ) and answer th...
Text Solution
|
- Consider the following experiments and answer the questions at the end...
Text Solution
|
- Consider the following experiments and answer the questions at the end...
Text Solution
|
- Consider the following experiments and answer the questions at the end...
Text Solution
|
- Consider the following experiments and answer the questions at the end...
Text Solution
|
- Two research students were instruced intructed to synthesise the compl...
Text Solution
|
- Two research students were instruced intructed to synthesise the compl...
Text Solution
|
- Two research students were instruced intructed to synthesise the compl...
Text Solution
|
- One cationic complex has to isomers A and B Each has one Co^(3+) five ...
Text Solution
|
- One cationic complex has to isomers A and B Each has one Co^(3+) five ...
Text Solution
|
- Complexes A and B have similarity in the following but not in .
Text Solution
|
- Velence bond theroy describes the bonding in complexs in terms of coor...
Text Solution
|
- Velence bond theroy describes the bonding in complexs in terms of coor...
Text Solution
|
- Give an example of displacement reaction.
Text Solution
|
- Square planar complexes are formed by d^(8) ions with strong field lig...
Text Solution
|
- Square planar complexes are formed by d^(8) ions with strong field lig...
Text Solution
|
- If in the mixed carbonyl the other ligand is also pi acceptor it would...
Text Solution
|