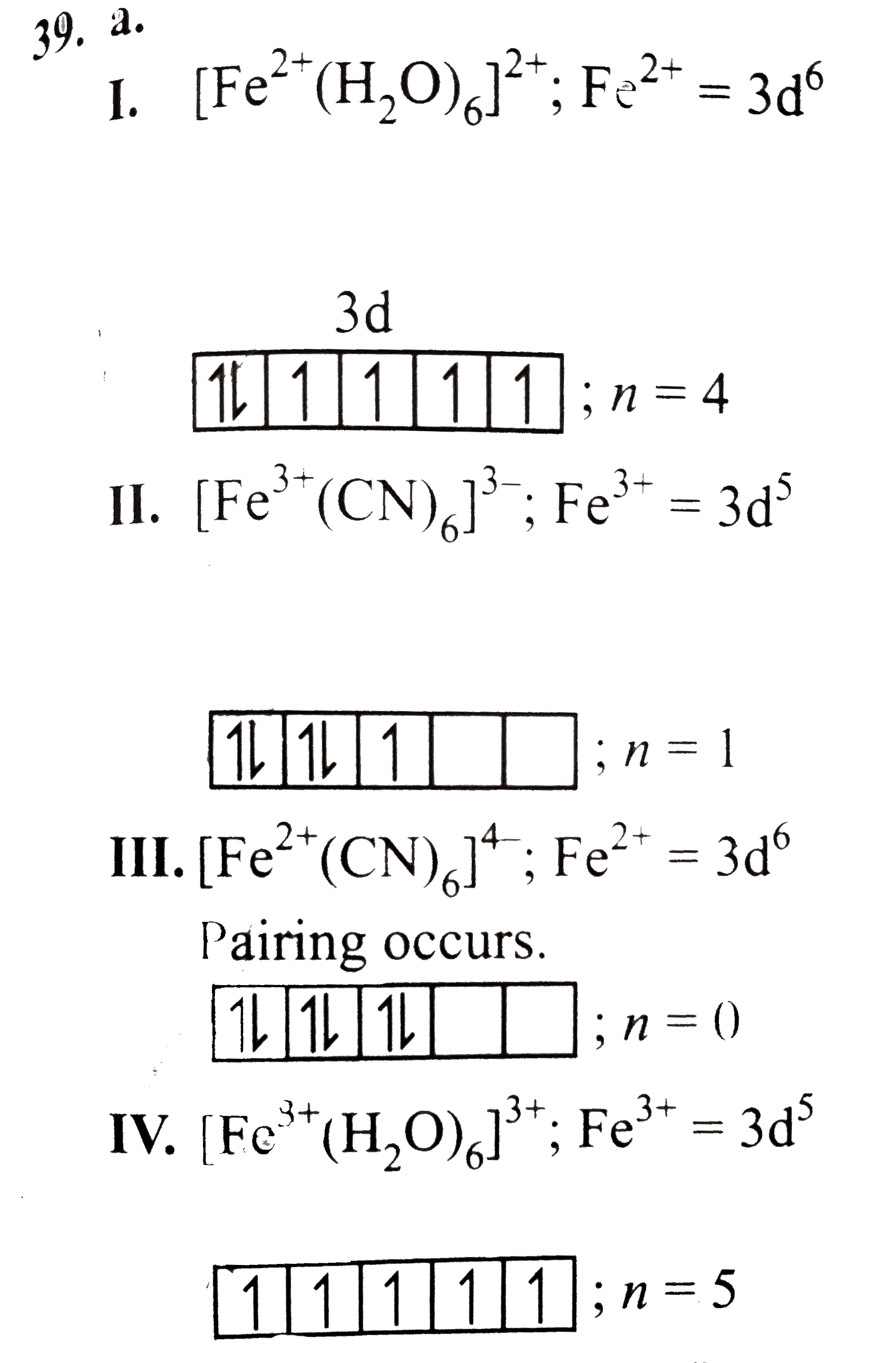Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct(Naming And Terminology)|9 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct(Isomerism )|10 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 7.2 Objective|8 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-COORDINATION COMPOUNDS-Exercises Linked Comprehension
- Square planar complexes are formed by d^(8) ions with strong field lig...
Text Solution
|
- If in the mixed carbonyl the other ligand is also pi acceptor it would...
Text Solution
|
- If in the mixed carbonyl the other ligand is also pi acceptor it would...
Text Solution
|
- If in the mixed carbonyl the other ligand is also pi acceptor it would...
Text Solution
|
- Most of the metal carbonyls obey inert gas rule which states the the c...
Text Solution
|
- If in the mixed carbonyl the other ligand is also pi acceptor it would...
Text Solution
|
- Most of the metal carbonyls obey inert gas rule which states the the c...
Text Solution
|
- In the manufacture of iron a gas (A) is formed in the zone of combusti...
Text Solution
|
- In the manufacture of iron a gas (A) is formed in the zone of combusti...
Text Solution
|
- The pi acceptor ligands are those which possess vacant pi- orbitals in...
Text Solution
|
- The pi acceptor ligands are those which possess vacant pi- orbitals in...
Text Solution
|
- The pi acceptor ligands are those which possess vacant pi- orbitals in...
Text Solution
|
- The pi acceptor ligands are those which possess vacant pi- orbitals in...
Text Solution
|
- The pi acid ligands donate their lone pairs to the metal to form a nor...
Text Solution
|
- The pi acid ligands donate their lone pairs to the metal to form a nor...
Text Solution
|
- On a frictionless horizontal surface , assumed to be the x-y plane ,...
Text Solution
|
- Coordination compounds plays many important roles in animals and plant...
Text Solution
|
- Coordination compounds plays many important roles in animals and plant...
Text Solution
|
- Coordination compounds plays many important roles in animals and plant...
Text Solution
|
- Arrange the following in order of decreasing number of unpaired electr...
Text Solution
|