Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Fill The Blanks|32 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises True/False|14 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Integer (Isomerism)|15 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-COORDINATION COMPOUNDS-Exercises Integer (Hybridisation , Vbt , Cft And Application)
- In hexacyanidomanganate(II) ion the Mn atom assumes d^(2)sp^(3)- hybri...
Text Solution
|
- Give the number of unpaired electron(s) in the complex ion[CoCI(6)]^(3...
Text Solution
|
- Predict the number of unpaired electrons in a tetrahedral d^(6) ion an...
Text Solution
|
- Give the number of unpaired electron present in the d-orbitals (whose ...
Text Solution
|
- Give the number of 3d electrons occupied in t(2g) orbitals of hydrated...
Text Solution
|
- How many unpaired electrons are present in e(g) orbital of MnO(4) Θ .
Text Solution
|
- How many electrons are present in d(z2) orbital of [Ni(gly)(2)] ? .
Text Solution
|
- Give the total number of t(2g) and e(g) electrons in [NiF(6)]^(2-) .
Text Solution
|
- How many electrons are present in d-orbitals which are present along t...
Text Solution
|
- If Hund' s rule is violated then how many unpaired electrons are prese...
Text Solution
|
- Give the number of unpaired electrons in t(2g) set of d-orbitals in [C...
Text Solution
|
- How many maximum atom (s)is//are are present in same plane of Cr(CO)(6...
Text Solution
|
- Find out the number of hydrogen bonds present in the structure of the ...
Text Solution
|
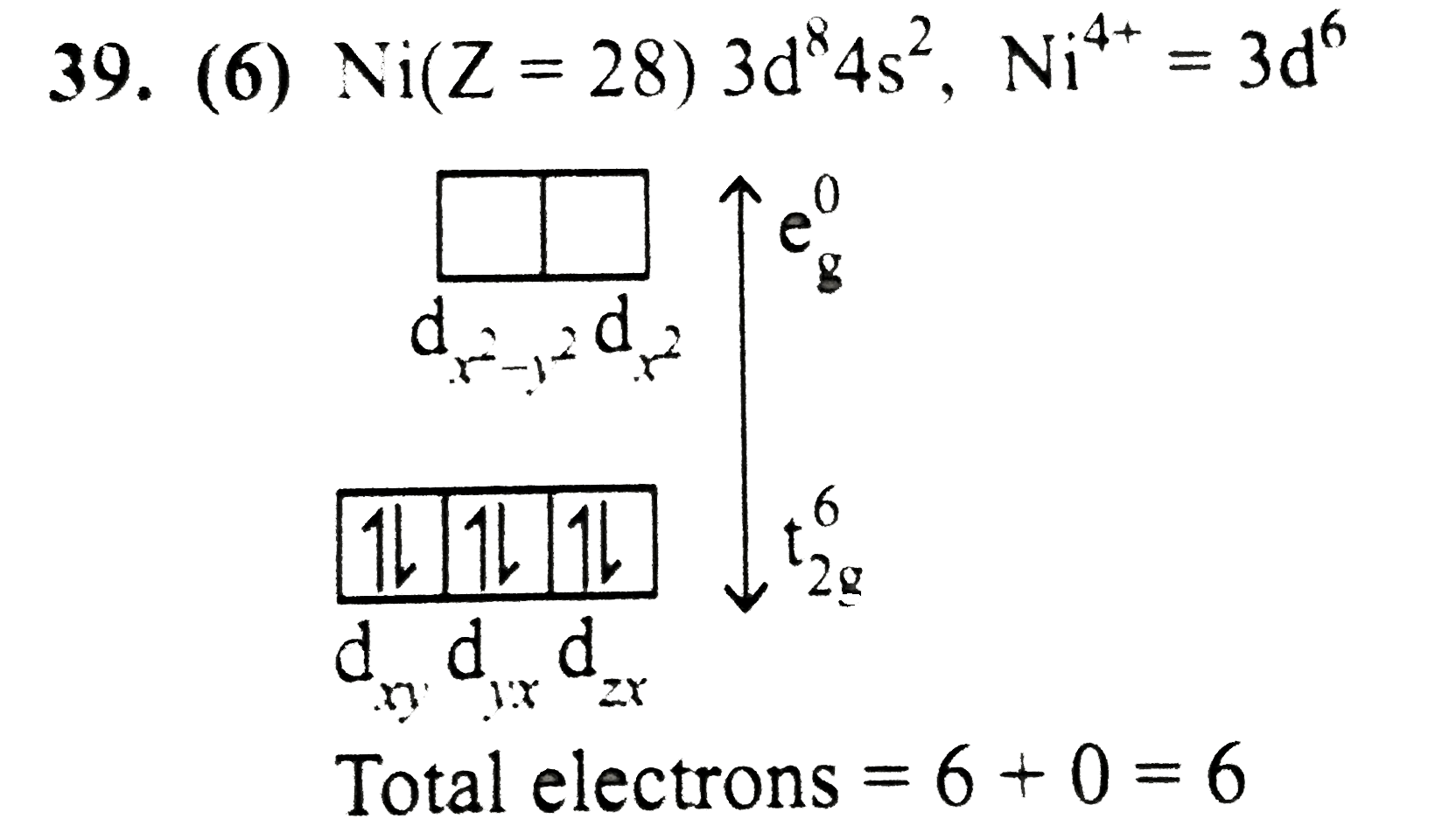 .
.