Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Multiple Correct|1 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Single Correct|28 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises True/False|14 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-COORDINATION COMPOUNDS-Archives (Linked Comprehension)
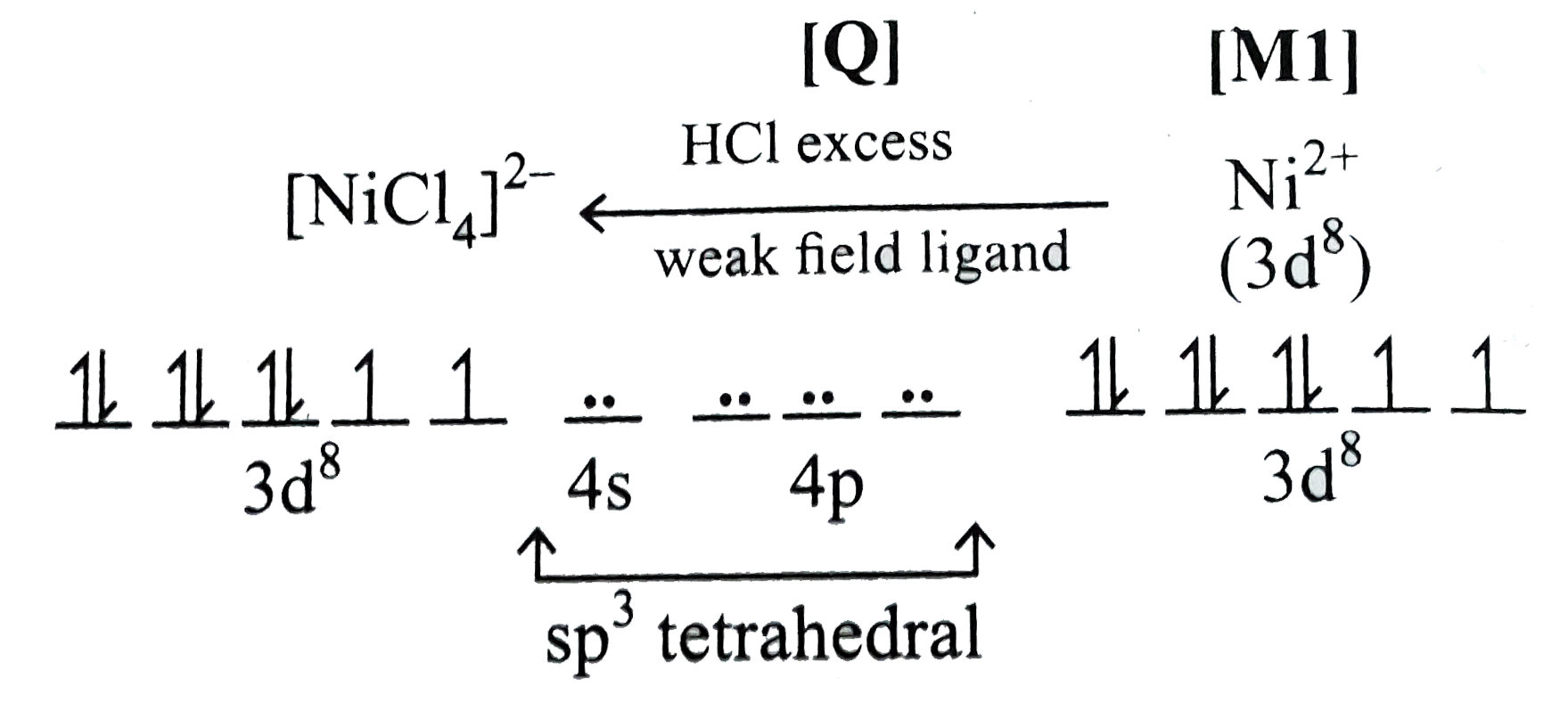
- The coordination number of Ni^(2+) is 4 NiCI(2) + KCN (excess)rarrA ...
Text Solution
|
- The coordination number of Ni^(2+) is 4 NiCI(2) + KCN (excess)rarrA ...
Text Solution
|
- The coordination number of Ni^(2+) is 4 NiCI(2) + KCN (excess)rarrA ...
Text Solution
|
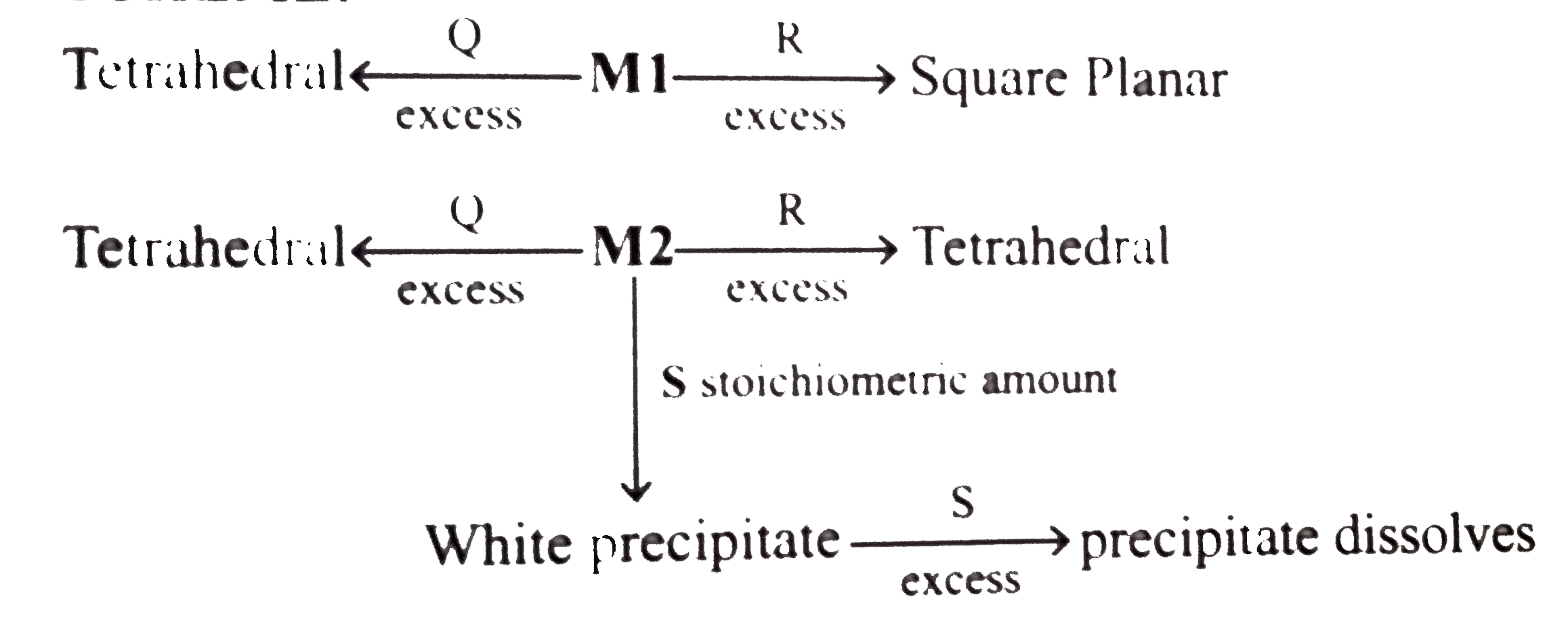
- An aqueous solution of metal ion MI reacts separately with reagents Q ...
Text Solution
|
- An aqueous solution of metal ion MI reacts separately with reagents Q ...
Text Solution
|