A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Assertion Reasoning|2 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Integer|2 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Multiple Correct|1 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-COORDINATION COMPOUNDS-Archives Single Correct
- The compound having a tetrahedral geometry is .
Text Solution
|
- Spin only magnetic moment in B.M. of the compound Hg(II)[Co(SCN)(4)] i...
Text Solution
|
- Which kind of isomerism is exhibited by the octahedral complex, [Co(NH...
Text Solution
|
- The bond length of C-O bond in carbon monoxide is 1.128A The C-O bond ...
Text Solution
|
- Among the following metal carbonyls, the bond order is lowest in :
Text Solution
|
- Both [Ni(CO)4] and [Ni (CN)4]^(2-) are diamagnetic The hybridisation...
Text Solution
|
- The IUPAC name of [Ni(NH3)4][NiCl4] is
Text Solution
|
- Among the following the coloured compound is
Text Solution
|
- The correct structure of ethylenediaminetetraacetic acid (EDTAH)
Text Solution
|
- The ionization isomer of [Cr(H2O)4Cl(NO2)]Cl is
Text Solution
|
- The complex showing a spin -magnetic momnet of 2.82 BM is .
Text Solution
|
- Geometrical shapes of the complexes formed by the reaction of Ni^(2+) ...
Text Solution
|
- Among the following complexes (K - P) : K(3) [Fe(CN)(6)] - K , [Co(NH(...
Text Solution
|
- As per IUPAC nomenclature, the name of the complex, [Co(H2O)4(NH3)2]Cl...
Text Solution
|
- The colour of light absobed by an aqueous solution of CuSO4 is
Text Solution
|
- [NiCI(2){P(C(2)H(5))(2)(C(6)H(5))}(2)] exhibits temperature dependent ...
Text Solution
|
- Which of the following complex species is not expected to exhibit opti...
Text Solution
|
- Consider the follwing complexes ion P,Q and R P =[FeF(6)]^(3-), Q=[V...
Text Solution
|
- If excess of AgNO(3) solution is added to 100 mL of a 0.024 M solution...
Text Solution
|
- The octahedral complex of a metal ion M^(3+) with four monodentate lig...
Text Solution
|
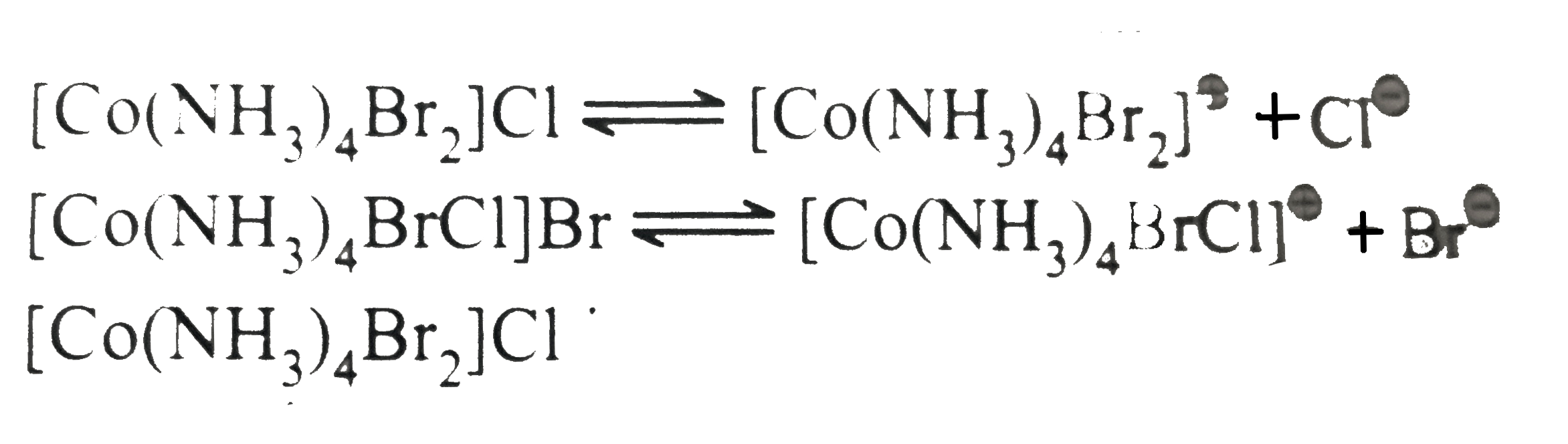 .
.