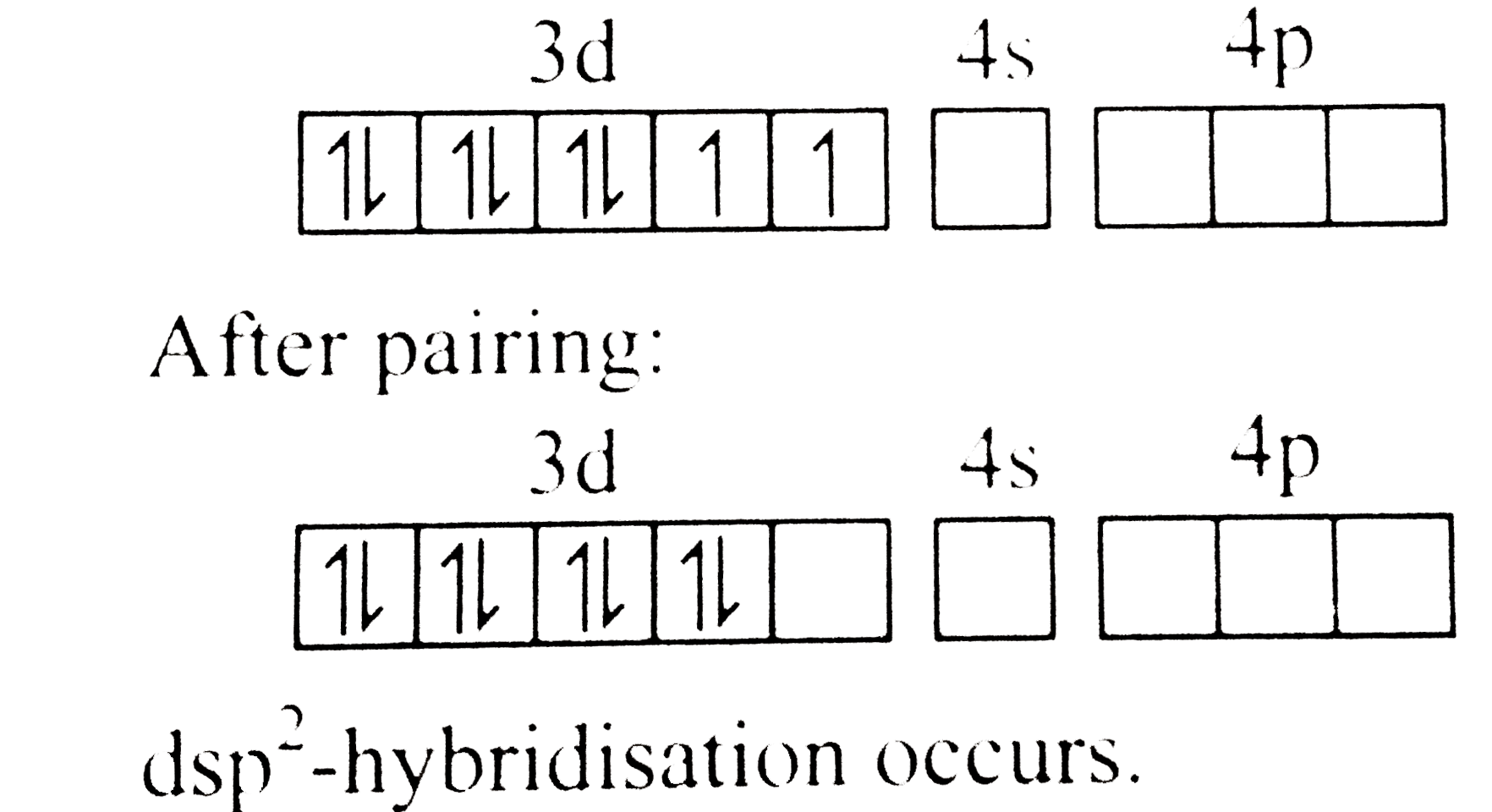
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-COORDINATION COMPOUNDS-Archives Subjective
- Write the balanced chemical equations for the following "Potassium fer...
Text Solution
|
- Give reasons in two or three sentences only for the following "The s...
Text Solution
|
- The acidic aqueous solution of ferrous ion forms a brown complex in th...
Text Solution
|
- Identify the complexes which are expected to be coloured. a. [Ti(NO(...
Text Solution
|
- Write the IUPAC name for the following compounds (a) [Co(NH(3))(5)O...
Text Solution
|
- Write the IUPAC name for [Cr(NH(3))(5)CO(3)]CI .
Text Solution
|
- Write a balanced equation for the reaction of argentite with KCN and n...
Text Solution
|
- Write the formulae of the following complexes (a) Pentamminechloroco...
Text Solution
|
- A,B and C are three complexes of chromium(III) with the empirical form...
Text Solution
|
- An aqueous solution containing one mole of HgI2 and two moles of NaI i...
Text Solution
|
- Draw the structures of [Co(NH(3))(6)]^(3+),[Ni(CN)(4)]^(2-) and [Ni(CO...
Text Solution
|
- A metal complex having composition Cr(NH(3))(4)CI(2)Br has been isolat...
Text Solution
|
- The magnitude of magnetic moment (spin only ) of [NiCl(4)]^(2-) will b...
Text Solution
|
- Write the IUPAC nomenclature of the given complex along with its hybr...
Text Solution
|
- NiCl(2) in the presence of dimethyl glyoxime (DMG) gives a complex wit...
Text Solution
|
- Aluminium trifluoride is insoluble in anhydrous HF but dissolves on ad...
Text Solution
|
- Write the balanced chemical equations for developing a black and white...
Text Solution
|
- Fe^(3+)overset(SNC^(Θ)("Excess"))rarrunderset("Bloodre"d)(A)overset(F^...
Text Solution
|