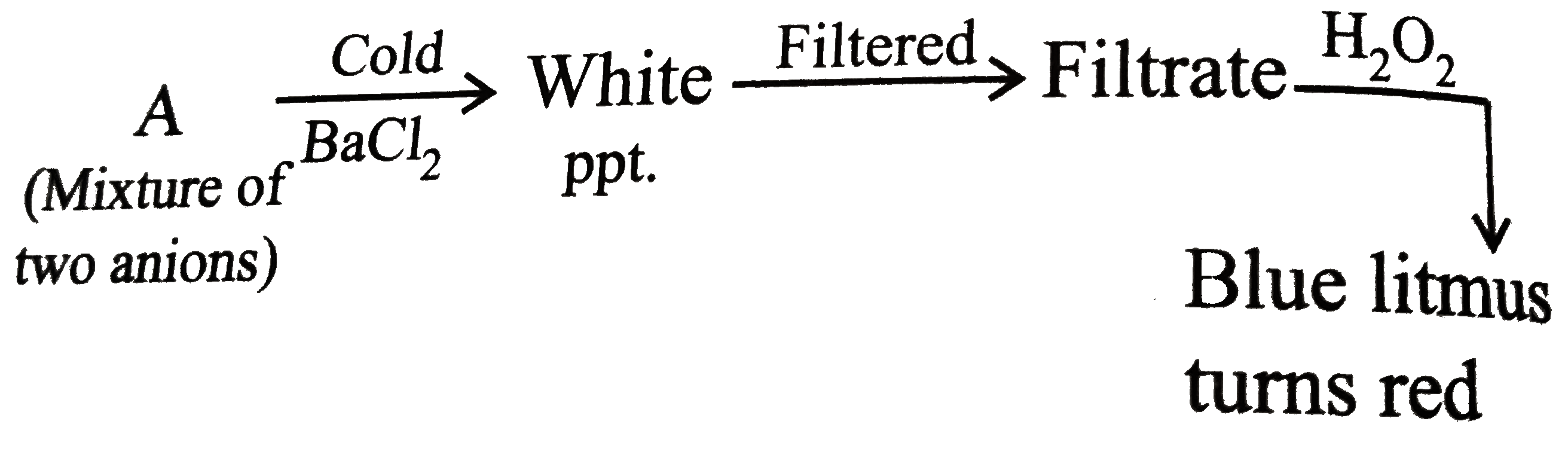A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct) Part-A (Analysis Of Anions)|24 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct) Part-B(Dry Test)|10 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct) Part-C(Dry Test)|8 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 (Objective)|14 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise SUBJECTIVE TYPE|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-QUALITATIVE INORGANIC SALT ANALYSIS-Exercises (Multiple Correct) Part-D (Miscellaneous)
- Select the correct statement(s):
Text Solution
|
- Mixture of A contains
Text Solution
|
- Which of the following statement (s) is/are incorrect ?
Text Solution
|
- A solution of colourless salt on boiling with excess NaOH produces a n...
Text Solution
|
- Which of the following statement is/are not true ?
Text Solution
|
- Which of the following react with dil H(2)SO(4)?
Text Solution
|
- Cone H(2)SO(4) will not give any gas with
Text Solution
|
- Select the correct statement(s):
Text Solution
|
- Select the correct statement(s):
Text Solution
|
- Select the correct statement(s):
Text Solution
|
- Select the correct statement(s):
Text Solution
|
- Which of the following complex(s) will have blue colour solution or pp...
Text Solution
|
- Which of the following statement(s) is/are with true ?
Text Solution
|
- Which of the following statement(s) is(are) correct ?
Text Solution
|
- Which of the following statement(s) is(are) incorrect ?
Text Solution
|
- Which of the following statement(s) is(are) correct ?
Text Solution
|
- Pick out the correct statement (s):
Text Solution
|
- Which of the following statement(s) is/are with true ?
Text Solution
|
- state true or false? Barium chromate is insoluble in dilute acetic aci...
Text Solution
|
- Potassium cyanide is used for separating
Text Solution
|