A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Viva Voce Questions And Part-A (Analysis Of Anions)|30 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Viva Voce Questions And Part-B (Dry Tests)|9 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 8.1|14 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 (Objective)|14 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise SUBJECTIVE TYPE|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-QUALITATIVE INORGANIC SALT ANALYSIS-Ex 8.2
- Aluminium sulphate (X) is slightly insoluble in water it is convert...
Text Solution
|
- CoCI(2) gives blue colour with NH(4)SCN due to formation of
Text Solution
|
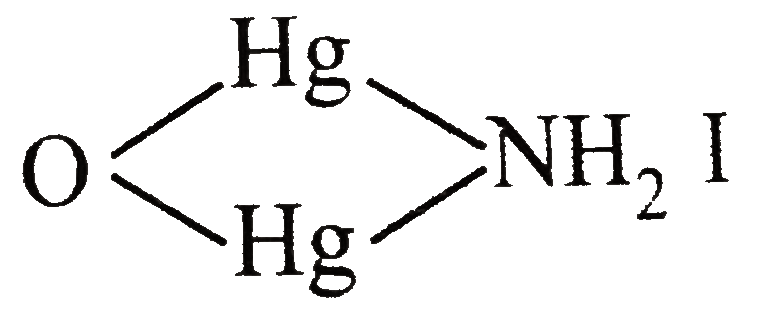
- HgCI(2)+ "excess of" KI rarr (A) underset(NH(3)) to (B) ,(A) and (B) ...
Text Solution
|
- NH(4)SCN can be used to test ion or more out of Fe^(3+) ,Co^(2+),Cu...
Text Solution
|
- K(4)[Fe(CN)(6)] can be used to detect one or more out of Fe^(2+)...
Text Solution
|
- Aqueous solution of borax reacts with two moles of acid. This is becau...
Text Solution
|
- Ag(2)S is soluble in NaCN due to formation of
Text Solution
|
- A compound give violet flame rest and gives a white ppt with AgNO(3...
Text Solution
|
- Bromine vapours turms ……..paper blue
Text Solution
|
- Solution of a salt in sulphanilic acid a naphithy lamine give red pp...
Text Solution
|
- Solution of a salt in dil H(2)SO(4) produces deep blue colour with st...
Text Solution
|
- The gas which turns mercurous nitrate paper black is
Text Solution
|
- A mixture when heated with dil H(2)SO(4) does not evolve brown vapour...
Text Solution
|
- To solution of a salt in acid medium AgNO(3) is added a white ppts r...
Text Solution
|
- Nitrite and nitrite both respond to ring test Nitrate are removed b...
Text Solution
|
- Which of the following metal oxide is white in colour but become ...
Text Solution
|
- Chromyl chloride test is preformed for the detection of CI^(Theta)...
Text Solution
|
- The chromyl chloride test responds poorly with the chlorides of Pb,Ag...
Text Solution
|
- When a salt is heated with dil H(2)SO(4) and KMnO(4) solution the pi...
Text Solution
|
- Ring test for mirates conformed by acidifying prepared FeSO(4) soi...
Text Solution
|