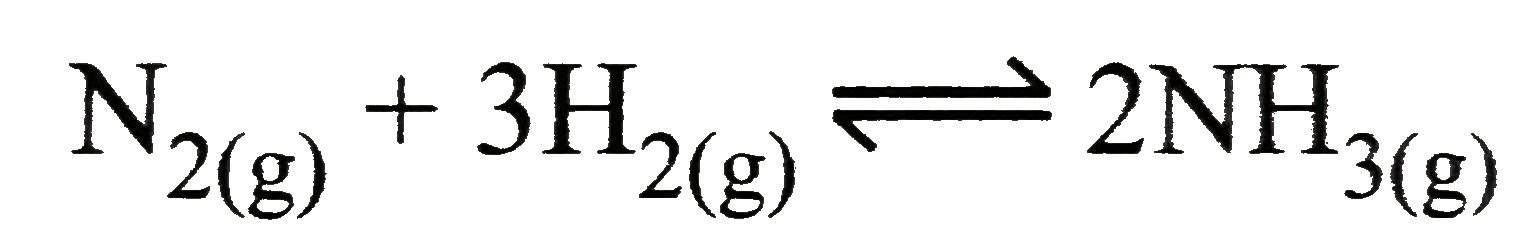Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises|137 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective Type|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-APPENDIX INORGANIC VOLUME 2-Short Answer Type
- Mention the conditions required to maximise the yield of ammonia
Text Solution
|
- Which metals are generally extracted by the electrolytic processes ? W...
Text Solution
|
- What type of ores are roasted?
Text Solution
|
- Give the names of the four most abundant elements in the earth's crust...
Text Solution
|
- What is a mineral? How does it differ from an ore?
Text Solution
|
- Why do some metals occur in the native state?
Text Solution
|
- Name the method used for refining of : (a). Zirconium. (b). Nickel...
Text Solution
|
- Can iron be purified by amalgamation?
Text Solution
|
- Both iron and aluminium combine slowly with oxygen at room temperature...
Text Solution
|
- What is the name given to solid phase of earth?
Text Solution
|
- Give one example where hydrometallurgy is used for metal extraction?
Text Solution
|
- Name the method of metal refining which is generally used when a metal...
Text Solution
|
- When is electrolytic reduction applied for getting a metal?
Text Solution
|
- What name is given to carbon reduction process for extracting the meta...
Text Solution
|
- An ore has impurities which are attracted by magnet suggest process fo...
Text Solution
|
- In moist air, copper corrodes to produce a green layer on the surface....
Text Solution
|
- The value ofDelta (f) G^(Ө) for formation of Cr(2) O(3) is – 540 kJmo...
Text Solution
|
- What do you understand by roasting in presence of NaCl?
Text Solution
|
- Why NF3 cannot be hydrolysed, while NCl3 can be readily hydrolysed?
Text Solution
|
- Why NI5 does not exist While PCl5 exists?
Text Solution
|
- Why the boiling point of ammonia is greater than phosphine?
Text Solution
|