Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective Type|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-APPENDIX INORGANIC VOLUME 2-Exercises
- Pb + conc. HNO(3) gives
Text Solution
|
- Give the resonating structures of NO(2) and N(2)O(5).
Text Solution
|
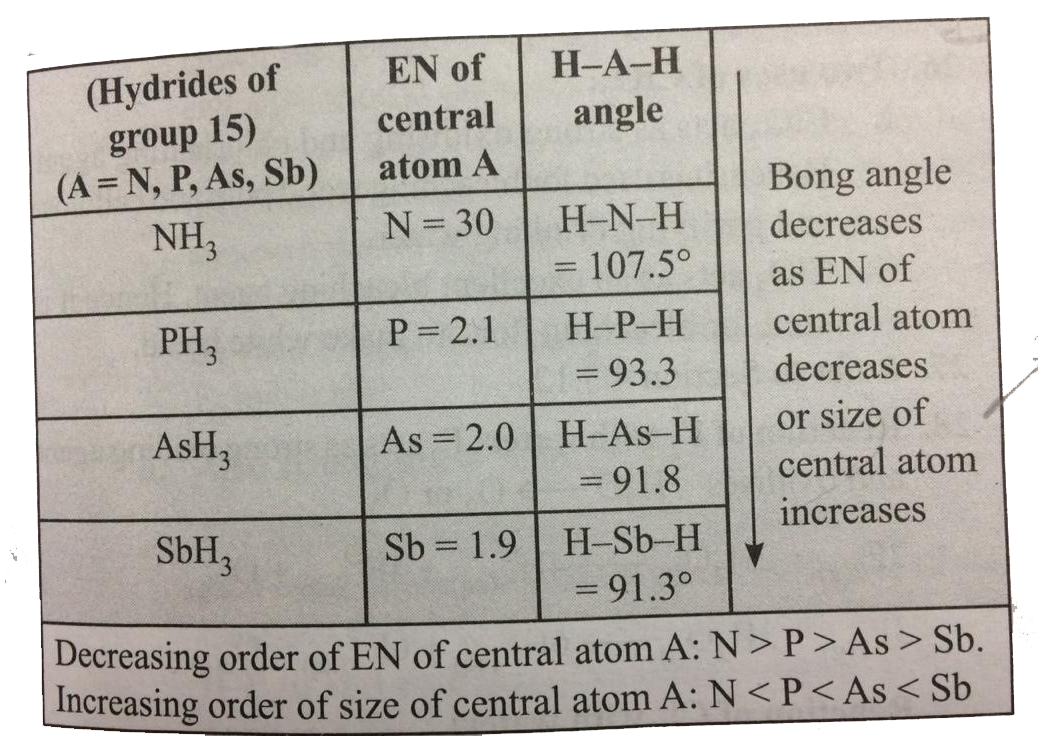
- The HNH angle value is higher than HPH, HAsH and HSbH angles. Why? ...
Text Solution
|
- Why does R(3)P = O exist but R(3)N = O does not (R = alkyl group)?
Text Solution
|
- Explain why NH(3) is basic while BiH3 is only feebly basic.
Text Solution
|
- Nitrogen exists as diatomic molecule and phosphorus as P(4). Why?
Text Solution
|
- Write main differences between the properties of white phosphorus and ...
Text Solution
|
- Why does nitrogen show catenation properties less than phosphorus ?
Text Solution
|
- Give the disproportionation reaction of H(3)PO(3).
Text Solution
|
- Can PCl(5) act as an oxidising as well as a reducing agent? Justify.
Text Solution
|
- Justify the placement of O, S, Se, Te and Po in the same group of the ...
Text Solution
|
- Why is dioxygen a gas but sulphur a solid?
Text Solution
|
- Knowing the electron gain enthalpy values for O to O^(-) and O to O^(...
Text Solution
|
- Which aerosols deplete ozone?
Text Solution
|
- Describe the manufacture of H(2)SO(4) by contact process?
Text Solution
|
- How is SO(2) an air pollutant?
Text Solution
|
- Why are halogens strong oxidising agents?
Text Solution
|
- Explain why fluorine forms only one oxoacid, HOF.
Text Solution
|
- Although chlorine and oxygen have nearly same electronegativity yet on...
Text Solution
|
- Write two uses of ClO(2).
Text Solution
|