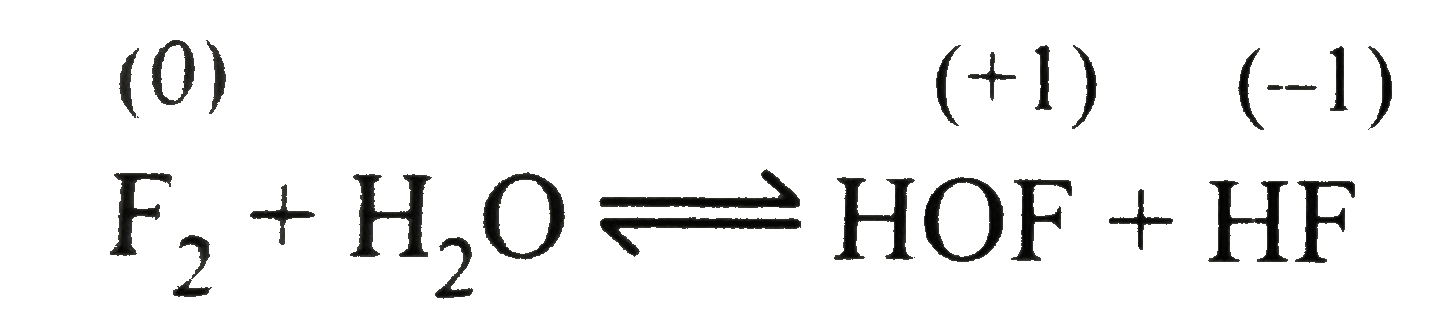Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective Type|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-APPENDIX INORGANIC VOLUME 2-Exercises
- How is SO(2) an air pollutant?
Text Solution
|
- Why are halogens strong oxidising agents?
Text Solution
|
- Explain why fluorine forms only one oxoacid, HOF.
Text Solution
|
- Although chlorine and oxygen have nearly same electronegativity yet on...
Text Solution
|
- Write two uses of ClO(2).
Text Solution
|
- Why are halogens coloured?
Text Solution
|
- Write the reactions of F(2) and Cl(2) with water.
Text Solution
|
- How can you prepare Cl(2) from HCl and HCl from Cl(2)? Write reactions...
Text Solution
|
- What inspired N. Bartlett for carrying out reaction between Xe and PtF...
Text Solution
|
- What are the oxidation states of phosphorus in the following:
Text Solution
|
- Write balanced equations for the following: (i) NaCl is heated wit...
Text Solution
|
- How are xenon fluorides XeF(2), XeF(4) and XeF(6) obtained?
Text Solution
|
- With what neutral molecule is ClO^(–) isoelectronic? Is that molecule ...
Text Solution
|
- How are XeO(3) and XeOF(4) prepared?
Text Solution
|
- Arrange the following in the order of property indicated for each set:
Text Solution
|
- Which one of the following does not exist?
Text Solution
|
- Give the formula and describe the structure of a noble gas which is is...
Text Solution
|
- Why do noble gases have comparatively large atomic sizes?
Text Solution
|
- List the uses of neon and argon gases.
Text Solution
|
- Write down the electronic configuration of: ltBrgt (i) Cr^(3+) (ii)...
Text Solution
|