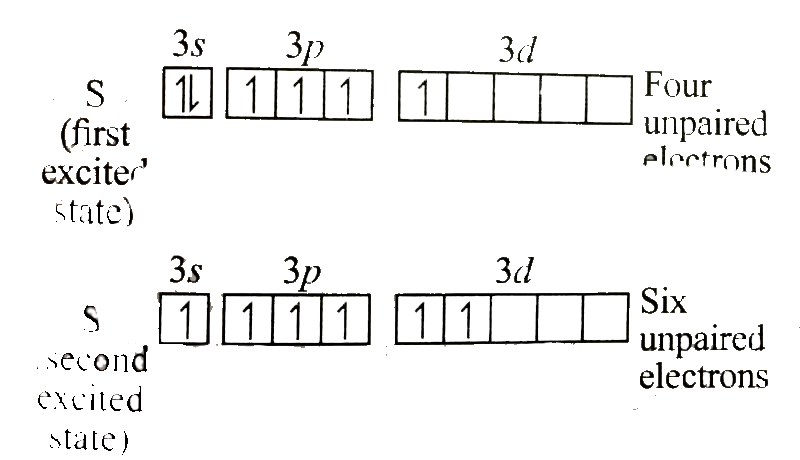
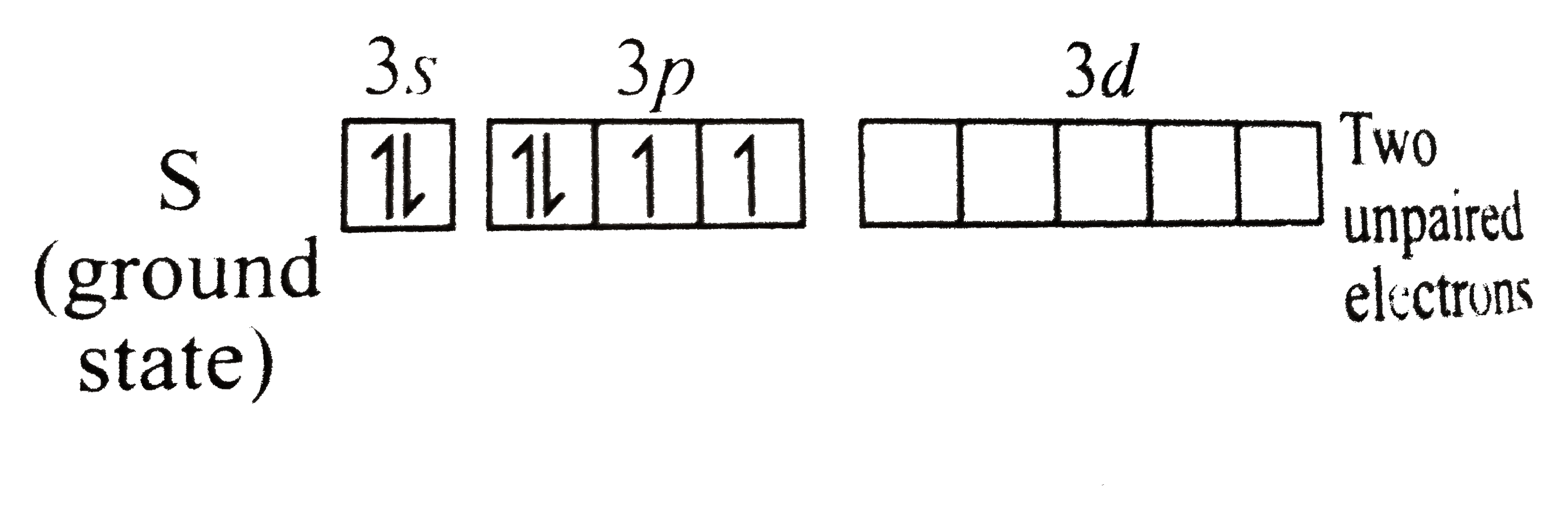Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises|137 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective Type|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-APPENDIX INORGANIC VOLUME 2-Short Answer Type
- Arrange the hydrised of group 16 in order of increasing bond angle.
Text Solution
|
- Write the formula of oxide, superoxide and peroxide of caesium.
Text Solution
|
- Why sulphur exhibits even oxidation states only?
Text Solution
|
- Arrange H2S,H2O,H2Se and H2Te in increasing order of reducing strength...
Text Solution
|
- What is the shape of SO3 molecule in gaseous state?
Text Solution
|
- Oxygen exhibits positive oxidation state in
Text Solution
|
- What is the atomicity of sulphur?
Text Solution
|
- What is the oxidation state of S in H2SO4?
Text Solution
|
- Name the hydride of group 16 elements which is liquid at room temperat...
Text Solution
|
- Which allotropic form of sulphur is most stable at room temperature?
Text Solution
|
- What is the maximum number of bonds formed by flourine?
Text Solution
|
- Name the halogens that can form true chemical bonds with noble gases?
Text Solution
|
- Name three gases which are used in warfare as posionous gases and prep...
Text Solution
|
- Name the most metalllic element among halogens?
Text Solution
|
- What is geometry of ClO2^(ɵ),ClO3^(ɵ) and ClO4^(ɵ) ions?
Text Solution
|
- Why are electron affinities of noble gases zero? Arrange halogens in i...
Text Solution
|
- Arrange the halongens in order of bond dissociation energies.
Text Solution
|
- Arrange the hydrogen halides in decreasing order of their reducing pow...
Text Solution
|
- What happens to acid strength among the acids of chlorine with an incr...
Text Solution
|
- The anydride of hypochlorous acid is ……………
Text Solution
|