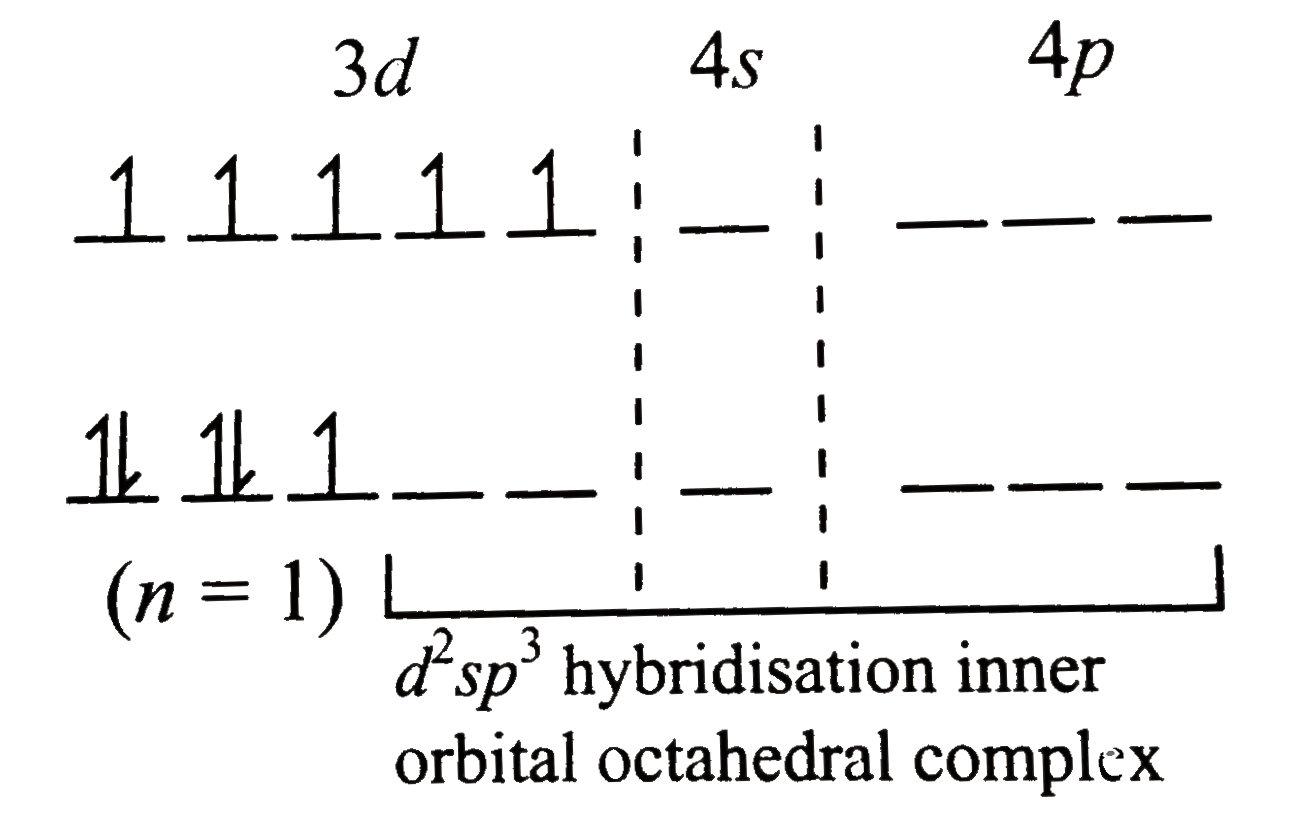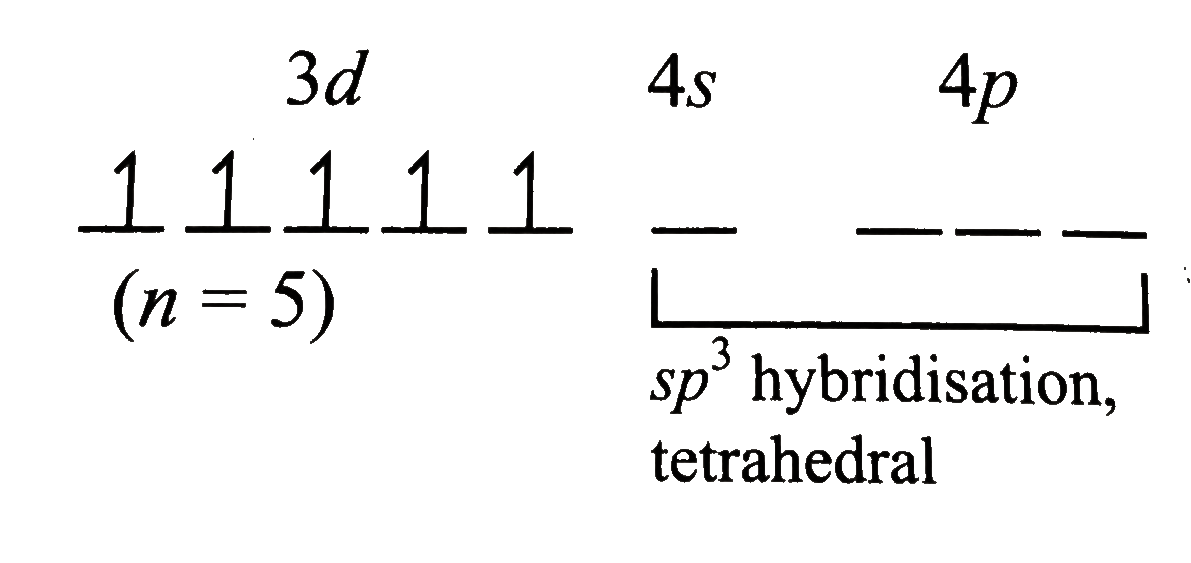Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective Type|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-APPENDIX INORGANIC VOLUME 2-Exercises
- Write down the number of 3d electrons in each of the following ions: ...
Text Solution
|
- Comment on the statement that elements of the first transition series ...
Text Solution
|
- What can be inferred from the magnetic moment values of the following ...
Text Solution
|
- Which of the following postulates of kinetic theory of gases is not co...
Text Solution
|
- FeSO(4) solution mixed with (NH(4))(2)SO(4) solution in 1:1 molar rati...
Text Solution
|
- Explain with two examples each of the following: coordination entity, ...
Text Solution
|
- What is meant by unidentate, didentate and ambidentate ligands? Give t...
Text Solution
|
- Specify the oxidation numbers of the metals in the following coordinat...
Text Solution
|
- Using IUPAC norms write the formulas for the following (i) Tetrahydr...
Text Solution
|
- Using IUPAC norms write the systematic names of the following: (i). ...
Text Solution
|
- List various types of isomerism possible for coordinatior compounds, g...
Text Solution
|
- How many geometrical isomers are possible in the following coordinatio...
Text Solution
|
- Draw the structures of optical isomers of: [Cr(C(2)O(4))(3)]^(3-) ...
Text Solution
|
- Draw the structures of optical isomers of: [Cr(C(2)O(4))(3)]^(3-) ...
Text Solution
|
- Write all the geometrical isomers of [Pt(NH(3))(Br)(Cl)(py)] and how m...
Text Solution
|
- Aqueous copper sulphate solution (blue in colour) gives: (i) a green p...
Text Solution
|
- What is the coordination entity formed when excess of aqueous KCN is a...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- The magnitude of CFSE (Crystal Field Splitting Energy , Delta(0)) can ...
Text Solution
|
- Write 1st 5 order and define What is spectrochemical series?
Text Solution
|