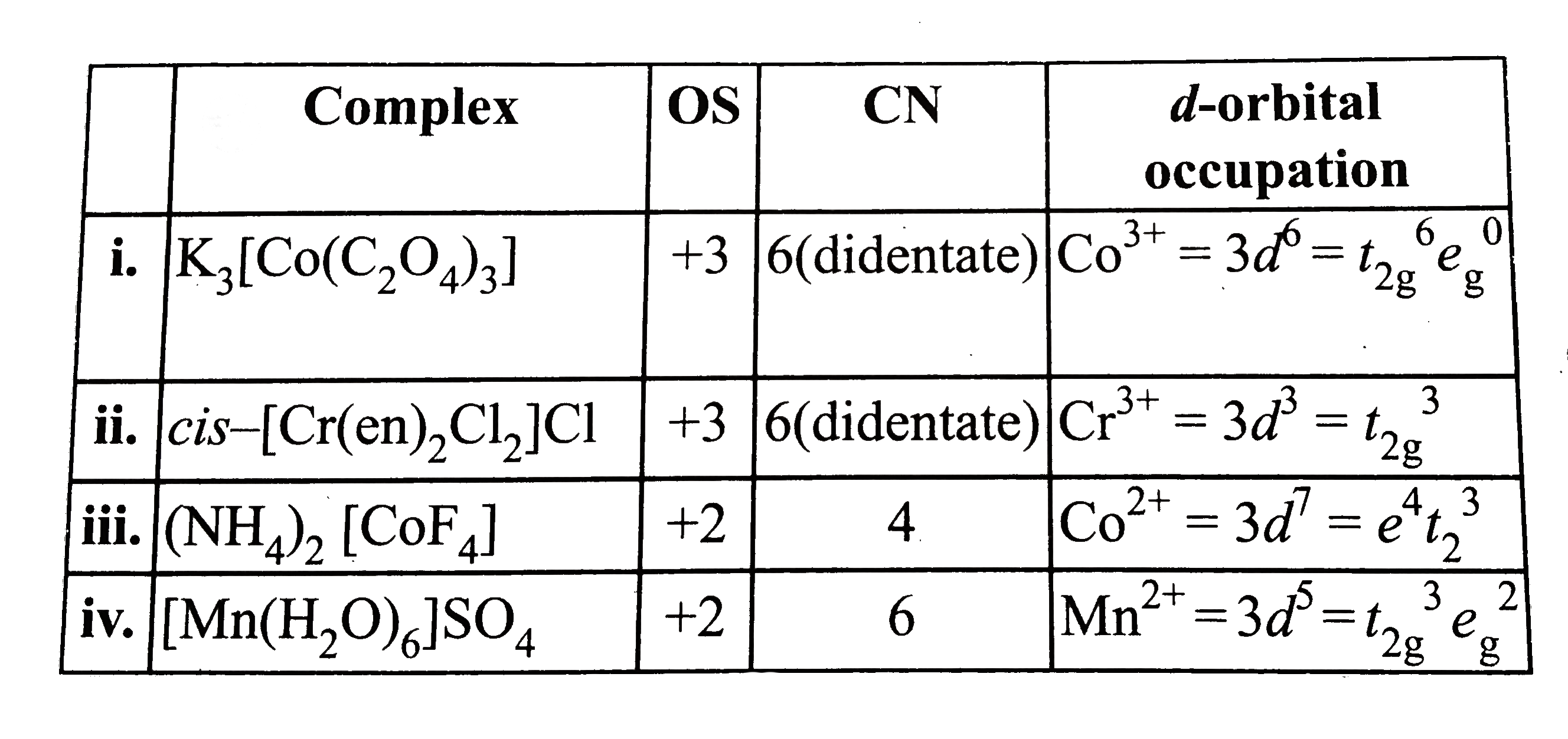Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective Type|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-APPENDIX INORGANIC VOLUME 2-Exercises
- Aqueous copper sulphate solution (blue in colour) gives: (i) a green p...
Text Solution
|
- What is the coordination entity formed when excess of aqueous KCN is a...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- The magnitude of CFSE (Crystal Field Splitting Energy , Delta(0)) can ...
Text Solution
|
- Write 1st 5 order and define What is spectrochemical series?
Text Solution
|
- The magnitude of CFSE (Crystal Field Splitting Energy , Delta(0)) can ...
Text Solution
|
- [Cr(NH3)6]^(3+) is paramagnetic while [Ni(CN)4]^(2-) is diamagnetic. E...
Text Solution
|
- A solution of [Ni(H(2)O)(6)]^(2+) is green but a solution of [Ni(CN)(4...
Text Solution
|
- [Fe(H(2)O)(6)]^(3+) is strongly paramagnetic whereas [Fe(CN)(6)]^(3-) ...
Text Solution
|
- Give the IUPAC name of the following compounds
Text Solution
|
- Give the oxidation state, d orbital occupation and coordination number...
Text Solution
|
- Write the IUPAC names of the following compounds
Text Solution
|
- STABILITY OF COORDINATION COMPOUND is of type
Text Solution
|
- What is meant by the chelate effect? Give an example.
Text Solution
|
- Discuss briefly giving an example in each case the role of coordinatio...
Text Solution
|
- How many ions are produced from the complex Co(NH3)4Cl2 in solution ? ...
Text Solution
|
- Amongst the following ions which one has the highest magnetic moment v...
Text Solution
|
- The oxidation number of hyrogen is (i) 0 (ii) +1 (iii) -1 ...
Text Solution
|
- Amongst the following, the most stable complex is (i) [Fe(H(2)O)(6)]...
Text Solution
|
- The correct order for the wavelength of absorption in the visible regi...
Text Solution
|