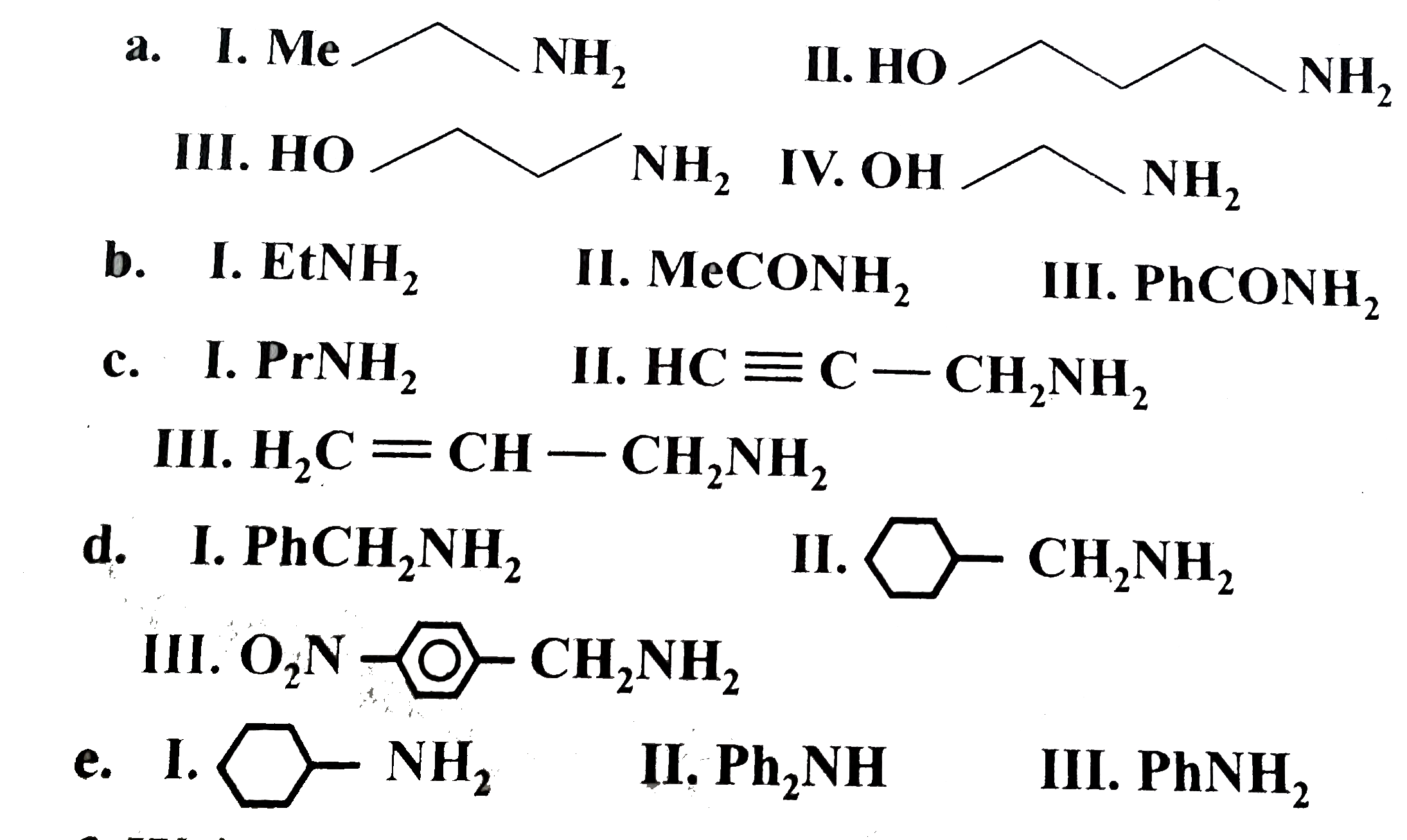
(a) `I gt II gt III gt Iv`
The `-I` effect of `OH` group decreases the `overline e` density on `N` atom, and thus decreases its basicity. If `- I` effect is large distance operating from `(-NH_2)`, then the base is more stronger, and vice versa. So, basicity `II gt III gt IV`.
(I) is the stronger base due to `+ I` effect of `(MeCH_2)` group.
(b) `I gt III gt II`
(i) The `+ I` effect of `(Et-)` group increases the `overline e - density` on `N` atom and thus increase basicity and is the stronger base.
(ii) `(C = O)` is `EWG` and decreases the basicity by delocalisation of `overline e-density` from `N` to `O`

Both the amides are weaker bases then amine. That is why amides amphoteric in nature, i., they are insoluble in aqueous acids and do not form salts unlike amines.
(iii) In benzamide `(PhCONH_2)`, there is cross conjugation of `(C = O)` with `Oh` group and with `NH_2` group, as shown below.
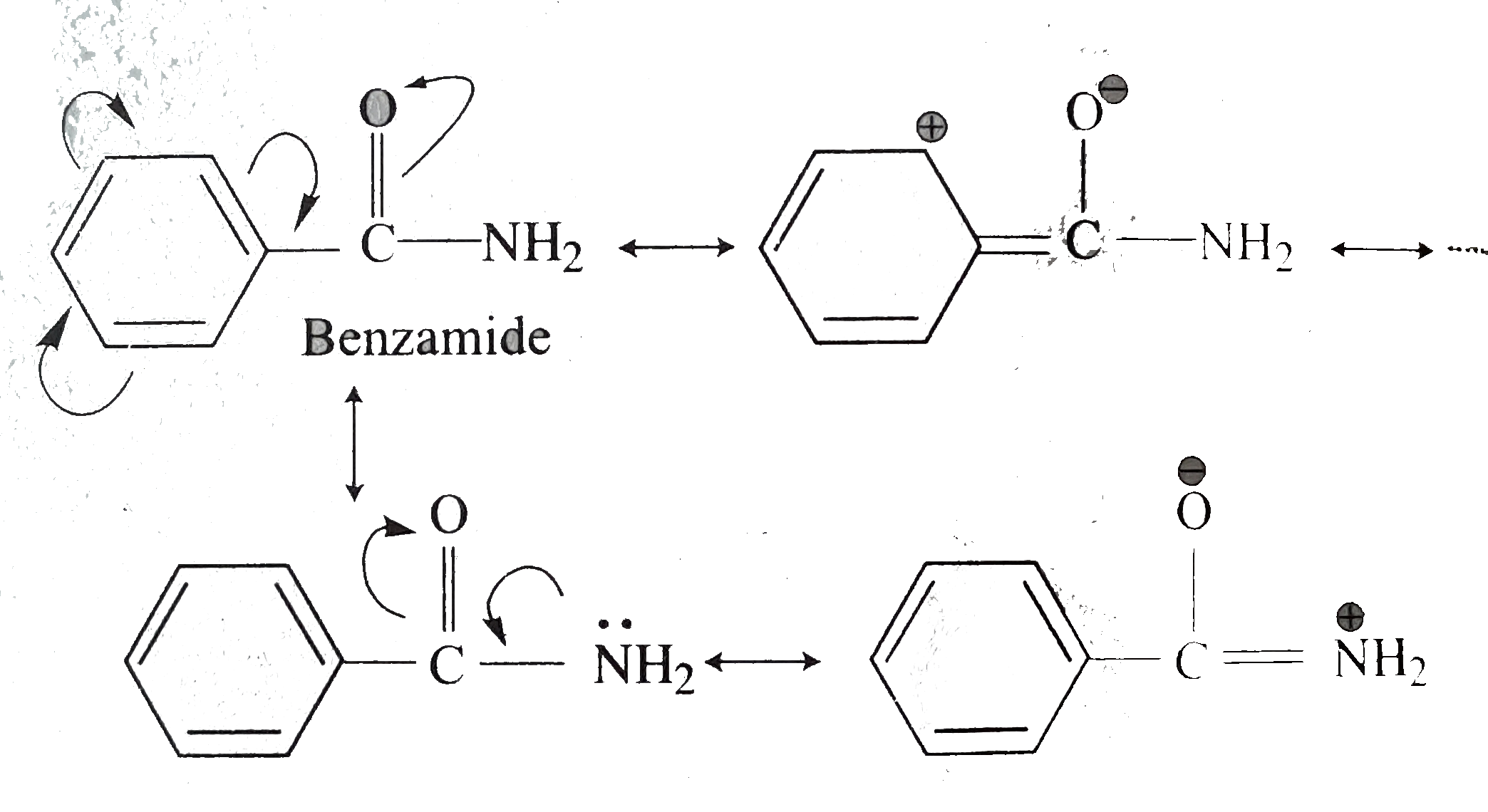
Therefore, `overline e-`density on `N` in benzamide (III) is slightly more than aliphatic amide (II). So, (III) is more basic than (II). Hencem basicity order is `EtNH_2 gt PhCONH_2 gt MeCONH_2`.
( c) State of hybridisation decides the basic character. Basic order is `sp^3 gt sp^2 gt sp` hybrid orbitals.
Conversly, the more the `s` character (as in `sp` hybrid orbitals) of `beta - ddot C` atom, the more is the `overline e-` withdrawing `-I` effect, and weaker the base. So, basicity order is :

Alternatively, `overline e `- withdrawing `- I` effect of :
`underset("Propargyl")(HC-=CH-CH_(2)-gt) underset(Allyl)(CH_(2)-=CH-CH_(2)- gt)`
`underset("Propyl")(CH_(3)-CH_(2)-CH_(2)-)` (d) `II gt I gt III`
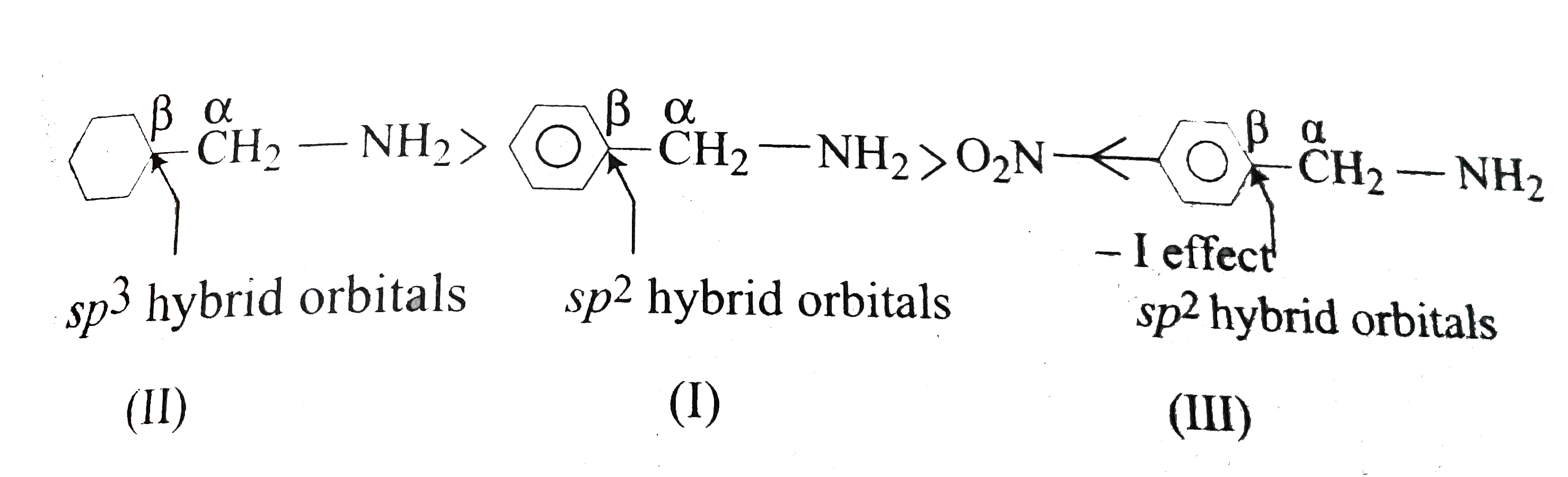
In (II), `beta-C` atom is `sp^3` atom is `sp^3` hybridised, whereas in (I) and (III), it is `sp^2` hybridised, so `overline e -` withdrawing - `I` effect of `sp^2 gt sp^3`. Moreover, `overline e-`withdrawing `p-NO_2` makes the `Ph` ring even more `overline e` withdrawing and decreasing the bascity.Hence, the order as given above.
( e) `I gt III gt II`
In (I)
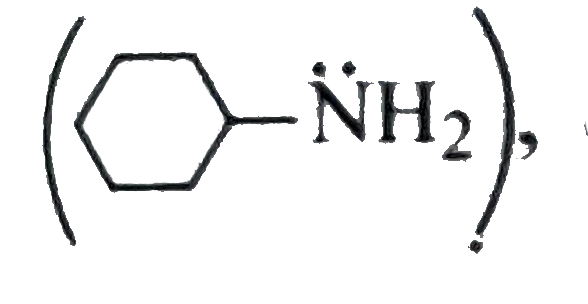
, `overline e-`density on `N` atom is localised and hence most basic.
In (II)
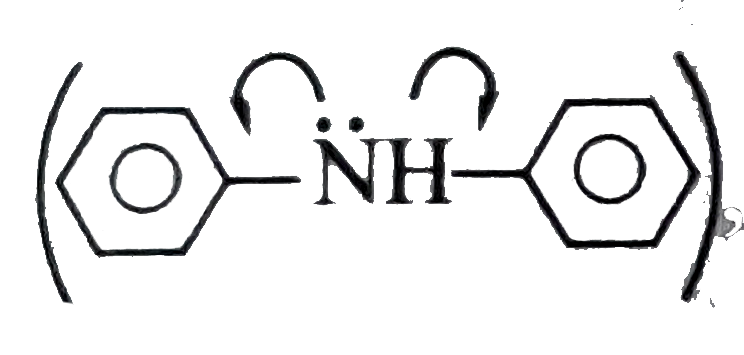
, `overline e-density` on `N` atom can be delocalised in both the `Ph` rings and hence least basic.
In (III)
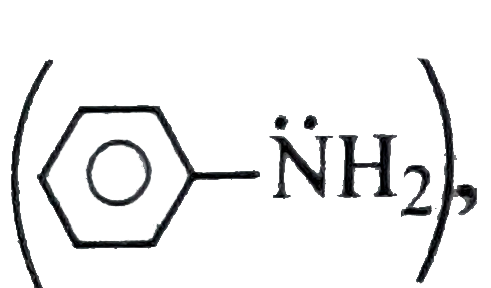
, `overline e- density` on `N` is delocalised only in `Ph` ring, and hence is less basic less than (I) and more basic than (II)
Moreover, (I) is an aliphatic (cycle) amine, and (II) and (III) are aromatic amines which are less basic than (I). In aromatic amines, `L P overline e^, s` on `N` atom are delocalised into the ring mainly at `o` and `p` positions, thereby weakening the basicity.
(f) In (I) `(HO - CH_2 - CH_2 - NH_2)`, is more acidic than `NH_2`. So, conjugate base of (I) is :
`O^(Ө) - CH_2 - CH_2 - NH_2`
(ii) In (I) is `HO - CH_2 - CH_2 - CH_2 - overset (oplus) N H_3`.



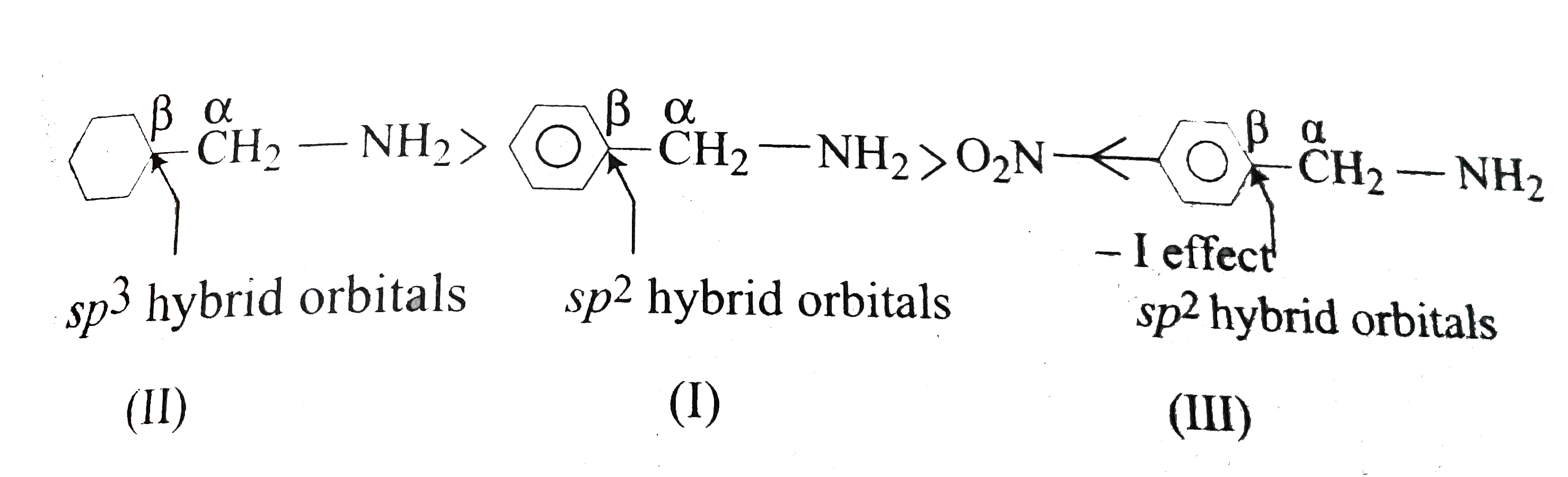
 , `overline e-`density on `N` atom is localised and hence most basic.
, `overline e-`density on `N` atom is localised and hence most basic.  , `overline e-density` on `N` atom can be delocalised in both the `Ph` rings and hence least basic.
, `overline e-density` on `N` atom can be delocalised in both the `Ph` rings and hence least basic.  , `overline e- density` on `N` is delocalised only in `Ph` ring, and hence is less basic less than (I) and more basic than (II)
, `overline e- density` on `N` is delocalised only in `Ph` ring, and hence is less basic less than (I) and more basic than (II)