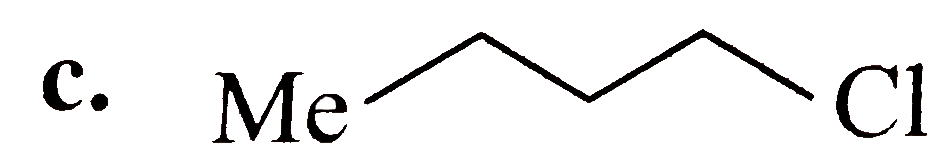
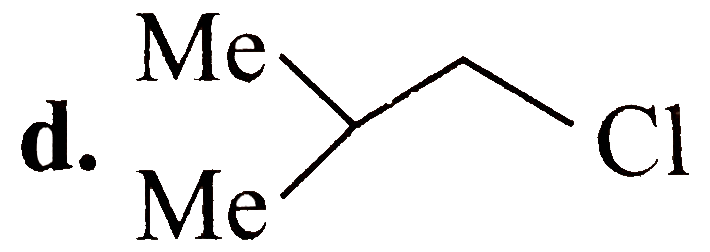
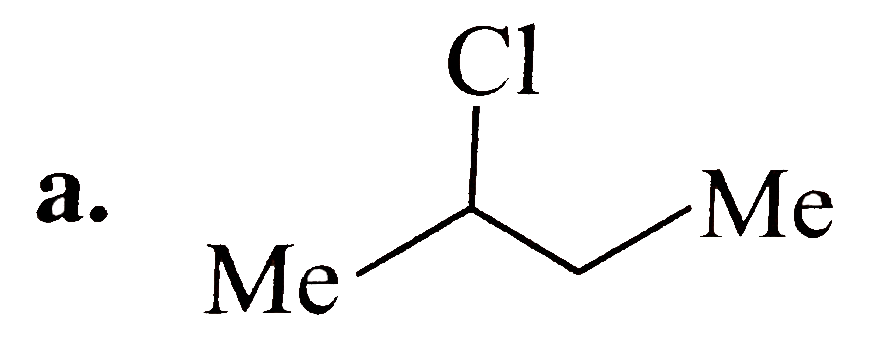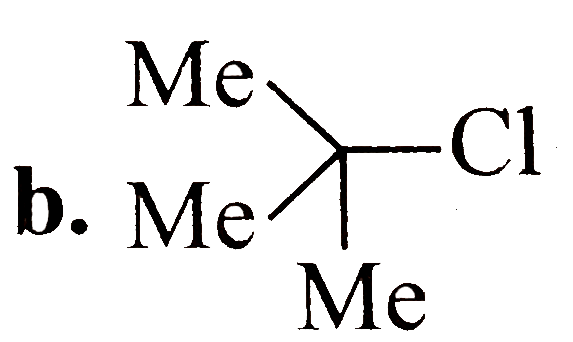A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning|12 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Archives|8 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple Correct|30 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise Archives (Subjecive)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC REACTION MECHANISM-Single correct
- Which of the following is the strongest nucleophile ?
Text Solution
|
- Rearrangement reactions are shown by :
Text Solution
|
- Equal amount of an RCl(C4 H9Cl) is reacted at the same temperature wit...
Text Solution
|
- Which of the following statements is correct about the following reac...
Text Solution
|
- Which statement is correct about the above reaction ?
Text Solution
|
- In SN^2, solvolysis RX in which the solvent is a nucleophile, what is ...
Text Solution
|
- A partially racemised (+) - 2-bromo-octane (2^@ RX) on reaction with a...
Text Solution
|
- Which of the compound in each of the following pairs will react faster...
Text Solution
|
- Which statement is correct about the following reactions ? .
Text Solution
|
- Which statement is correct about the following reactions ? .
Text Solution
|
- Which statement is correct about the following reactions ? .
Text Solution
|
- The decreasing order of dehydrohalogenation of the following compounds...
Text Solution
|
- The decreasing order of nucleophilicities of the following is : (I) ...
Text Solution
|
- The decreasing order of nucleophilicities of the following is : (I) ...
Text Solution
|
- The decreasing order of the basic character of the following is : (I...
Text Solution
|
- The decreasing order of nucleophilicities in DMSO (dimethylsulphoxide)...
Text Solution
|
- In which of the following reactions, SN^2 rate increases on changing t...
Text Solution
|
- In which of the following reactions, retention of configuration takes ...
Text Solution
|
- Necessary conditions for Fiels-Alder reactions are :
Text Solution
|
- Which of the following compounds would undergo Diels-Alder reaction wi...
Text Solution
|