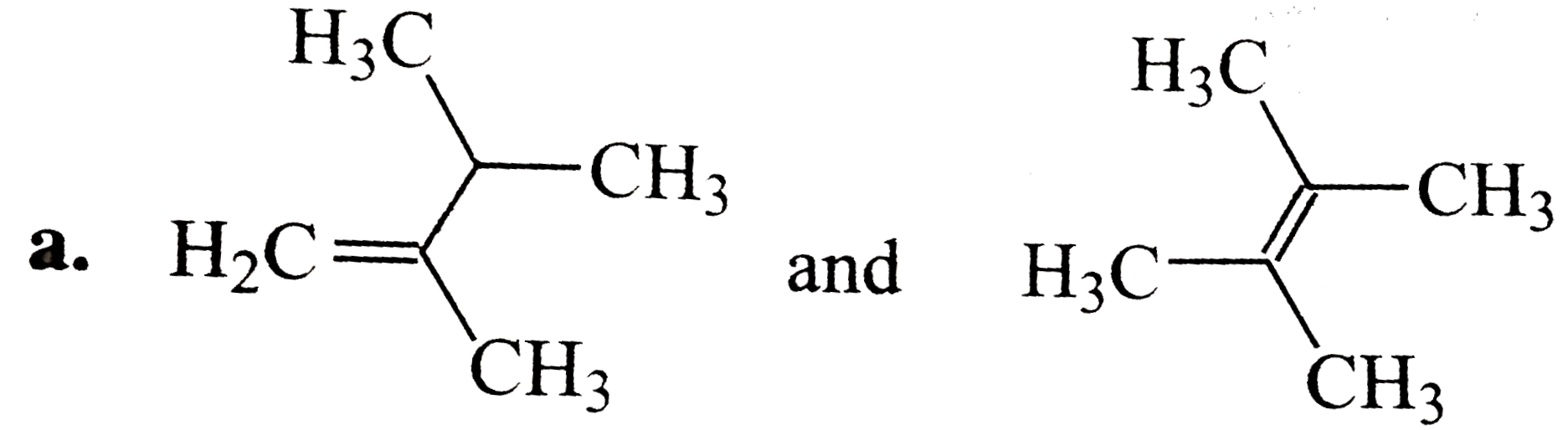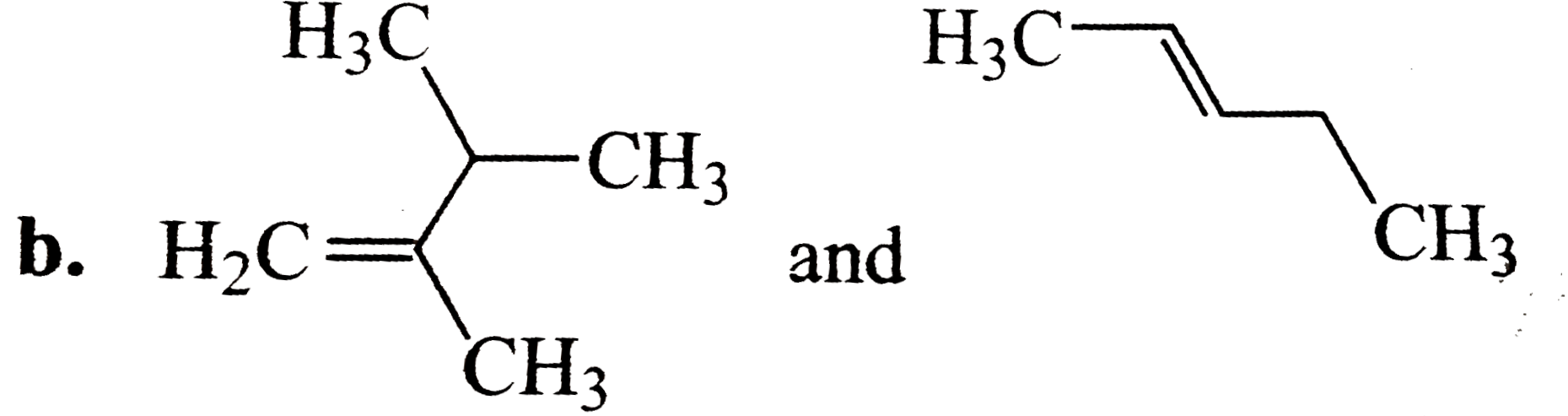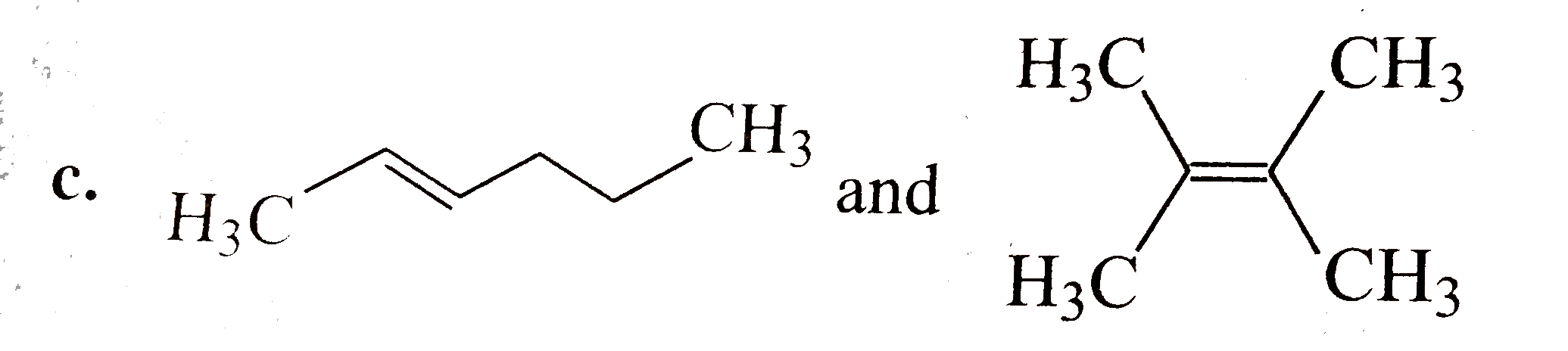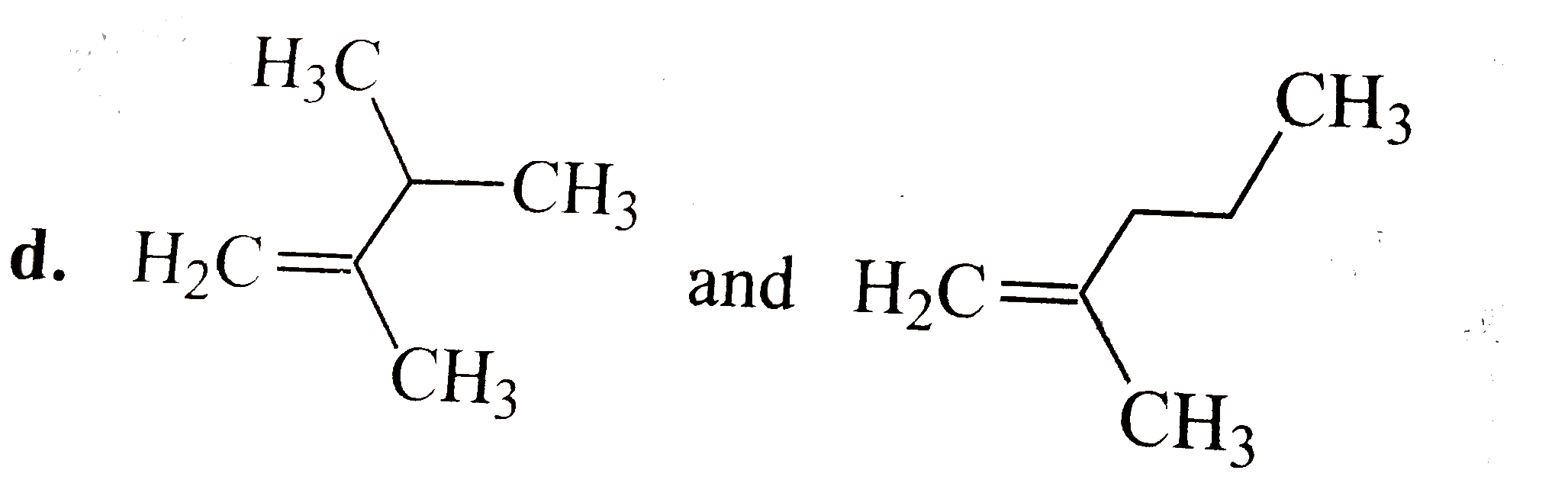A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning|12 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Archives|8 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple Correct|30 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise Archives (Subjecive)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC REACTION MECHANISM-Single correct
- The decresing order of reactivity towards SE (substitution by electrop...
Text Solution
|
- Which of the following is a free radical susbtitution reaction ?
Text Solution
|
- Reagent I and II, respectively, are :
Text Solution
|
- Which product is racemic in the above reaction ?
Text Solution
|
- Consider the following reactions.
Text Solution
|
- Which of the following is a non-aromatic compound ?
Text Solution
|
- The decreasing order of reactivity towards ArSN reaction of the follow...
Text Solution
|
- Consider the following reaction : The product is :
Text Solution
|
- Refer to Q.No. 38 and find out which of the following statements is w...
Text Solution
|
- Which of the following is not a rearrangement reaction ?
Text Solution
|
- Arrange the following in the reactions order of NA (nucleophilic addit...
Text Solution
|
- Arrange the following in the decreasing order of nucleophilic acyl sub...
Text Solution
|
- Which of the following reactions will not give Hofmann alkene ?
Text Solution
|
- Which of the following is the best method for the preparation of compo...
Text Solution
|
- Which statement is correct about the above reaction ?
Text Solution
|
- An alkyl halide of formula C6 H13 Br on treatment with potassium t-but...
Text Solution
|
- (A) and (B) are :
Text Solution
|
- Which of the following reactions would give trans-alkene ? .
Text Solution
|
- Which of the following reactions is Hofmann elimination ? .
Text Solution
|
- Draw the structure of the following compounds: cyclopropane.
Text Solution
|