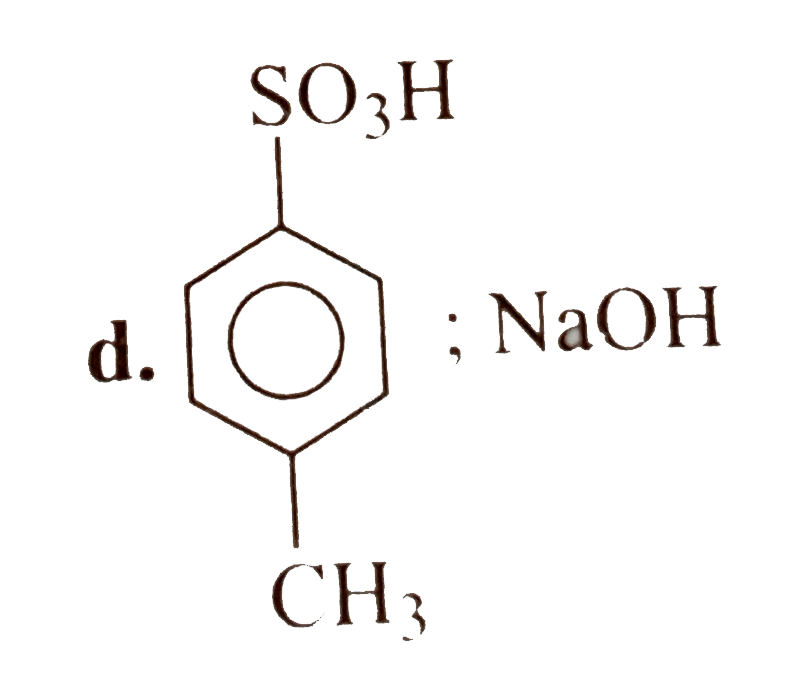
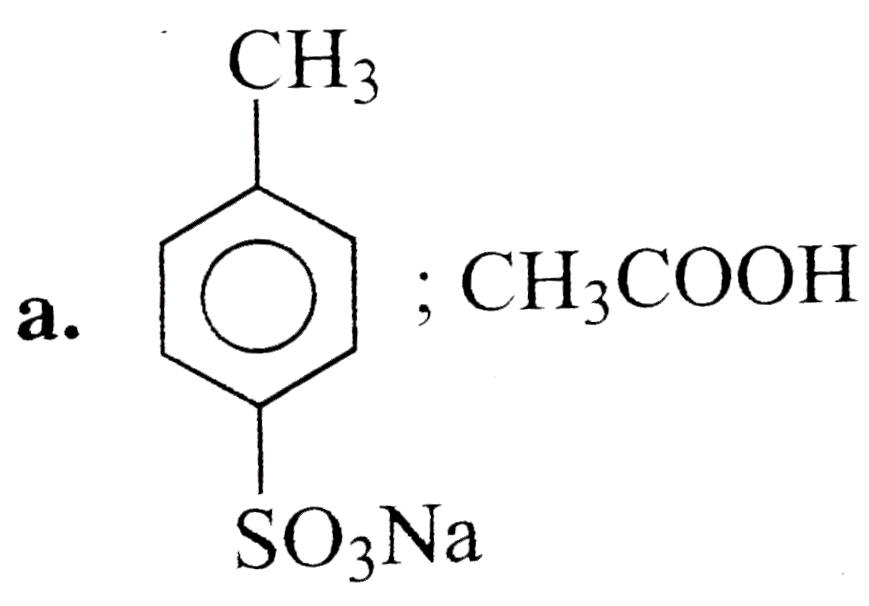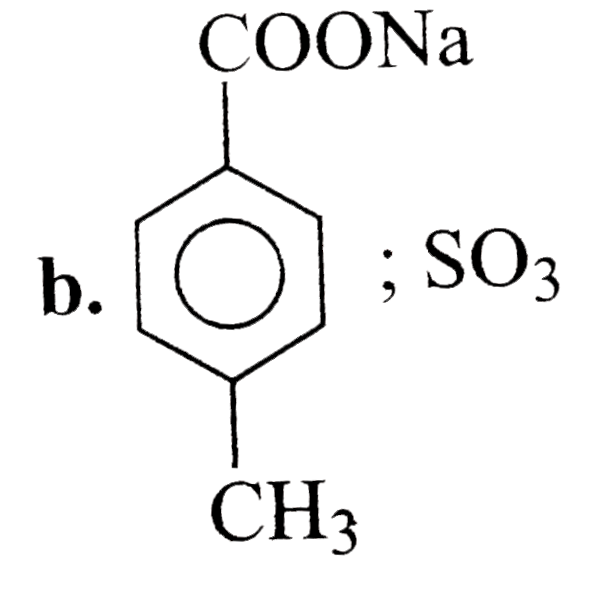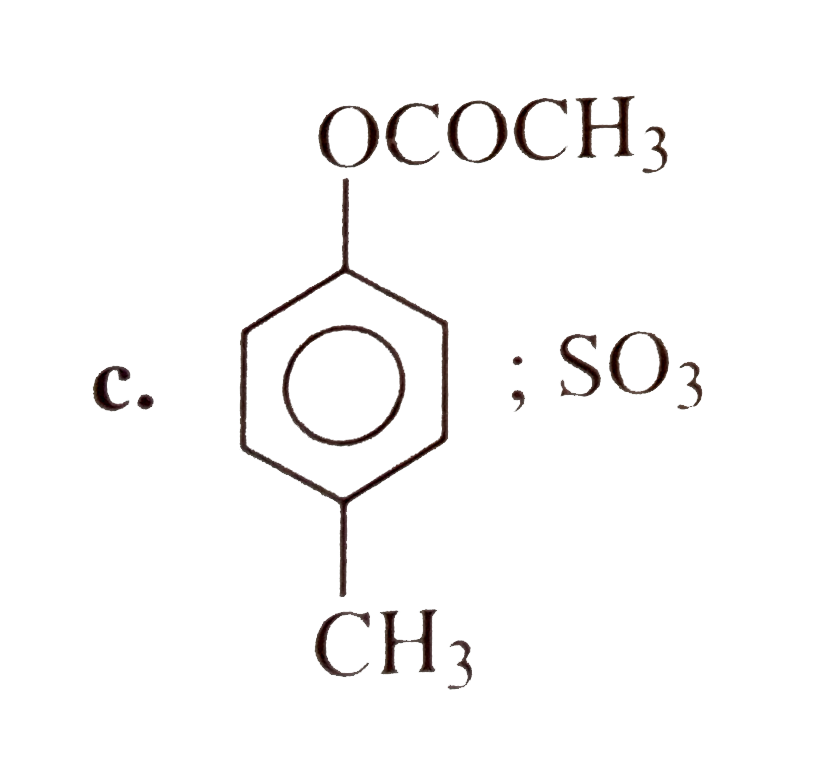A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise True/False|2 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|6 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning|12 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise Archives (Subjecive)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC REACTION MECHANISM-Archives
- The formation of cyanohydrin from a ketone is an example of nucleophi...
Text Solution
|
- Which of the following has the highest nucleophilicity ? F^(-) OH^...
Text Solution
|
- An S(N)2 reaction at an asymmetric carbon of a compound always g...
Text Solution
|
- Identify the correct of reactivity in electrophilic substitution react...
Text Solution
|
- 4-Methyl benzene sulphoic acid reacts with sodium acetate to give :
Text Solution
|
- The number of stereoisomers obtained by bromination of trans-2-butene ...
Text Solution
|
- The order of leaving group ability is :
Text Solution
|
- During debromination of meso-dibromobutane, the major compound formed ...
Text Solution
|