Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALKANES AND CYCLOALKANES-Archives
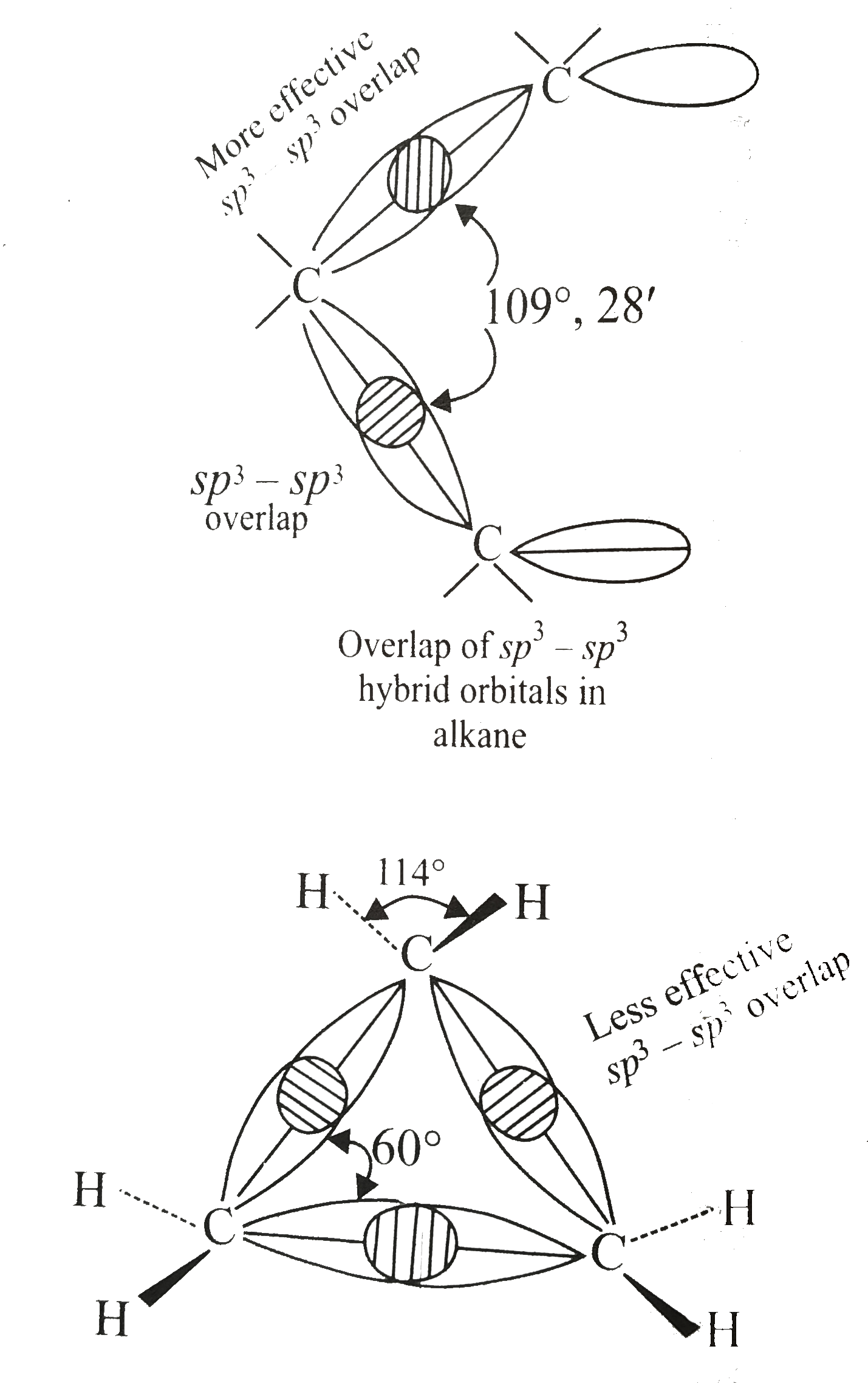
- Compare the orbital overlap in cyclopropane and alkane.
Text Solution
|
- Marsh gas mainly contains:
Text Solution
|
- The compound with the highest boiling point is:
Text Solution
|
- Highest boiling point is expected for
Text Solution
|
- The compound which has one isopropyl group is 2, 2, 3, 3-tetramethyl...
Text Solution
|
- C-H bond distance is the longest in :
Text Solution
|
- When cyclohexane is poured in water, it floats because:
Text Solution
|
- The products obtained at cathode and anode on electrolysis of aqueous ...
Text Solution
|
- Consider the following reaction: Identify the structure of the ma...
Text Solution
|
- How many chiral compounds are possible on monochlorination of 2-methyl...
Text Solution
|
- What would be the product formed when 1-bromo-3-chlorocylobutane rea...
Text Solution
|
- mu observed = Sigma mu(i)X(i) where mu(i) is the dipole moment of th...
Text Solution
|
- The value of N and M are:
Text Solution
|
- The total number of cyclic structure as well as stereoisomers possib...
Text Solution
|