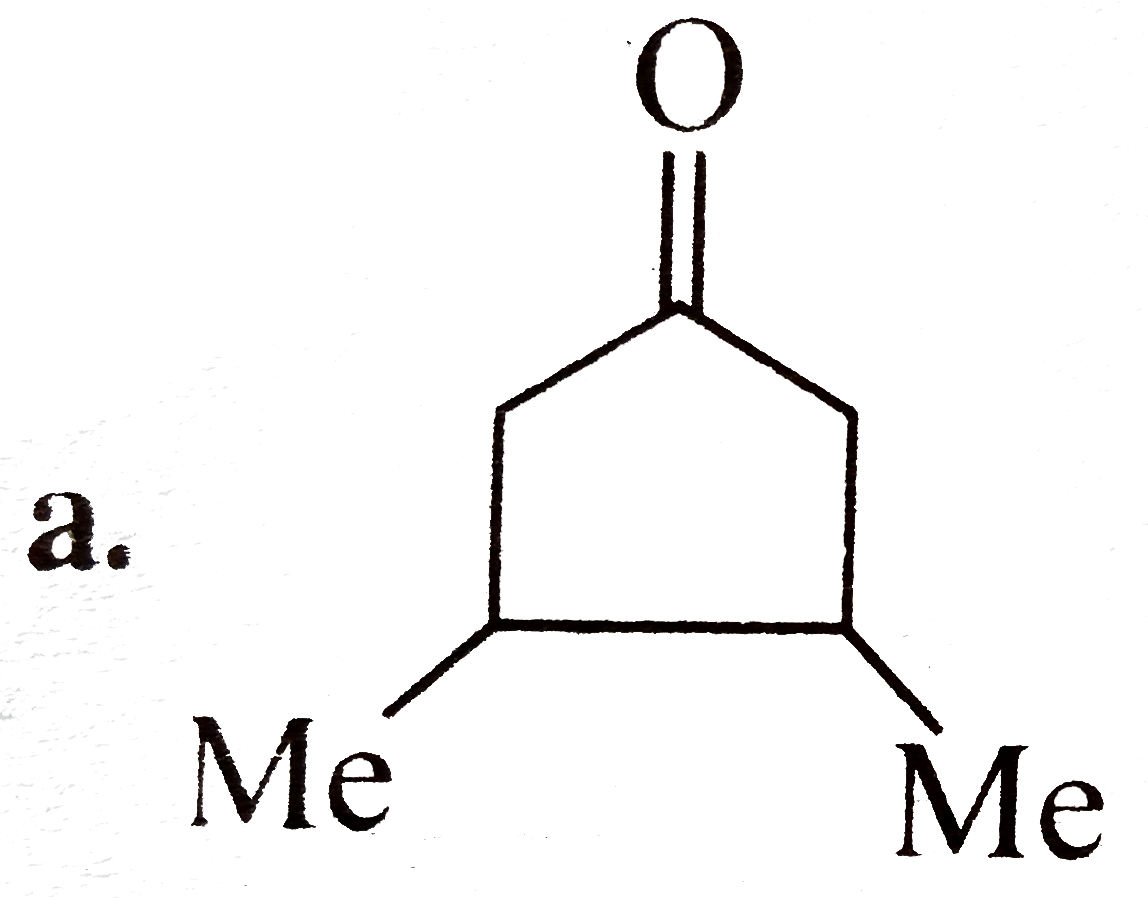
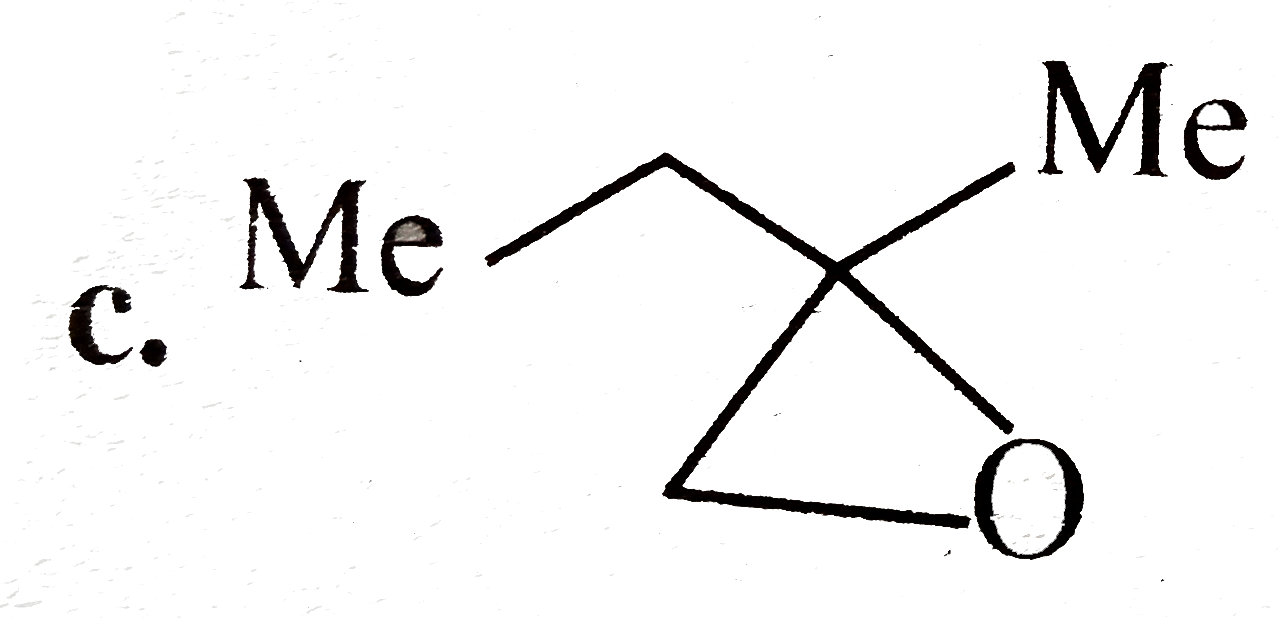
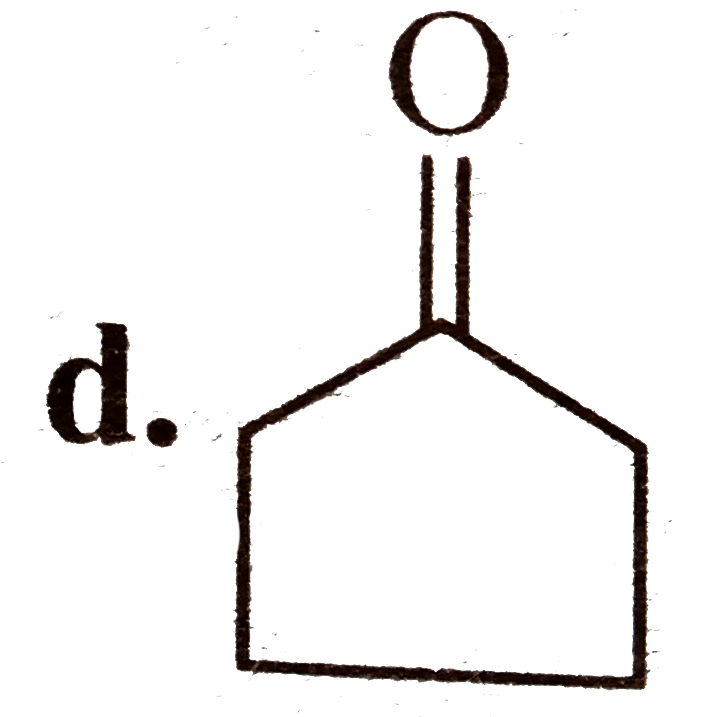
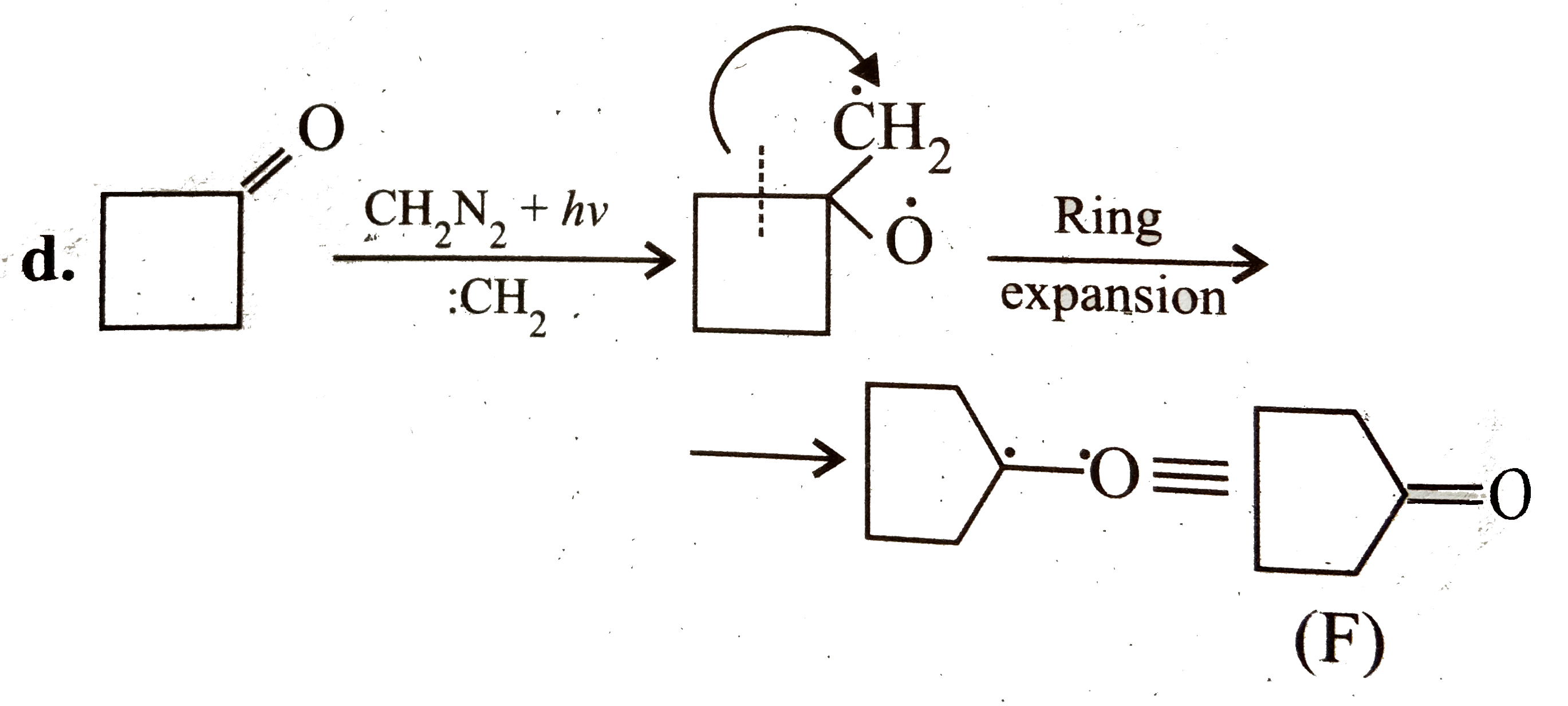
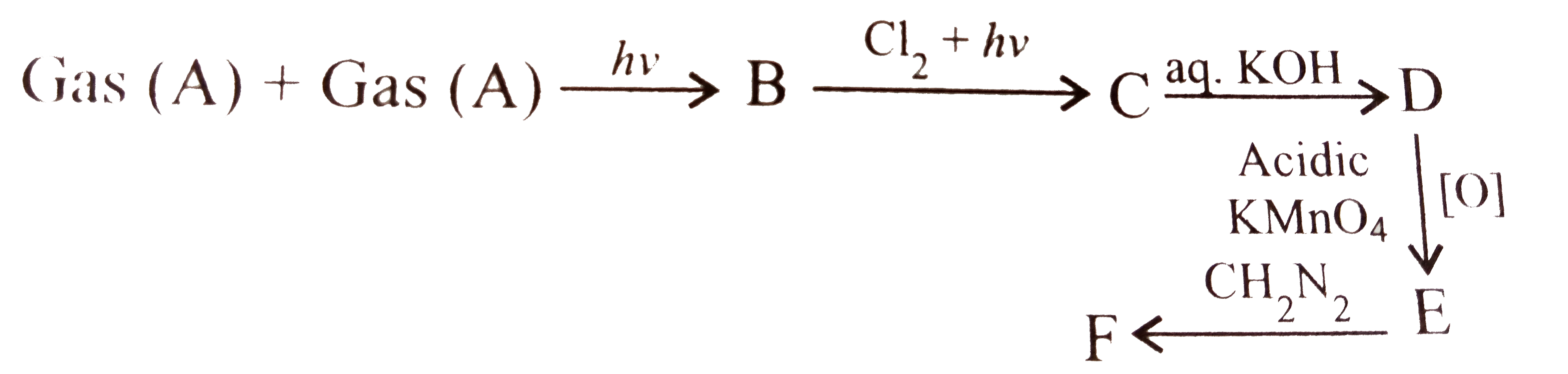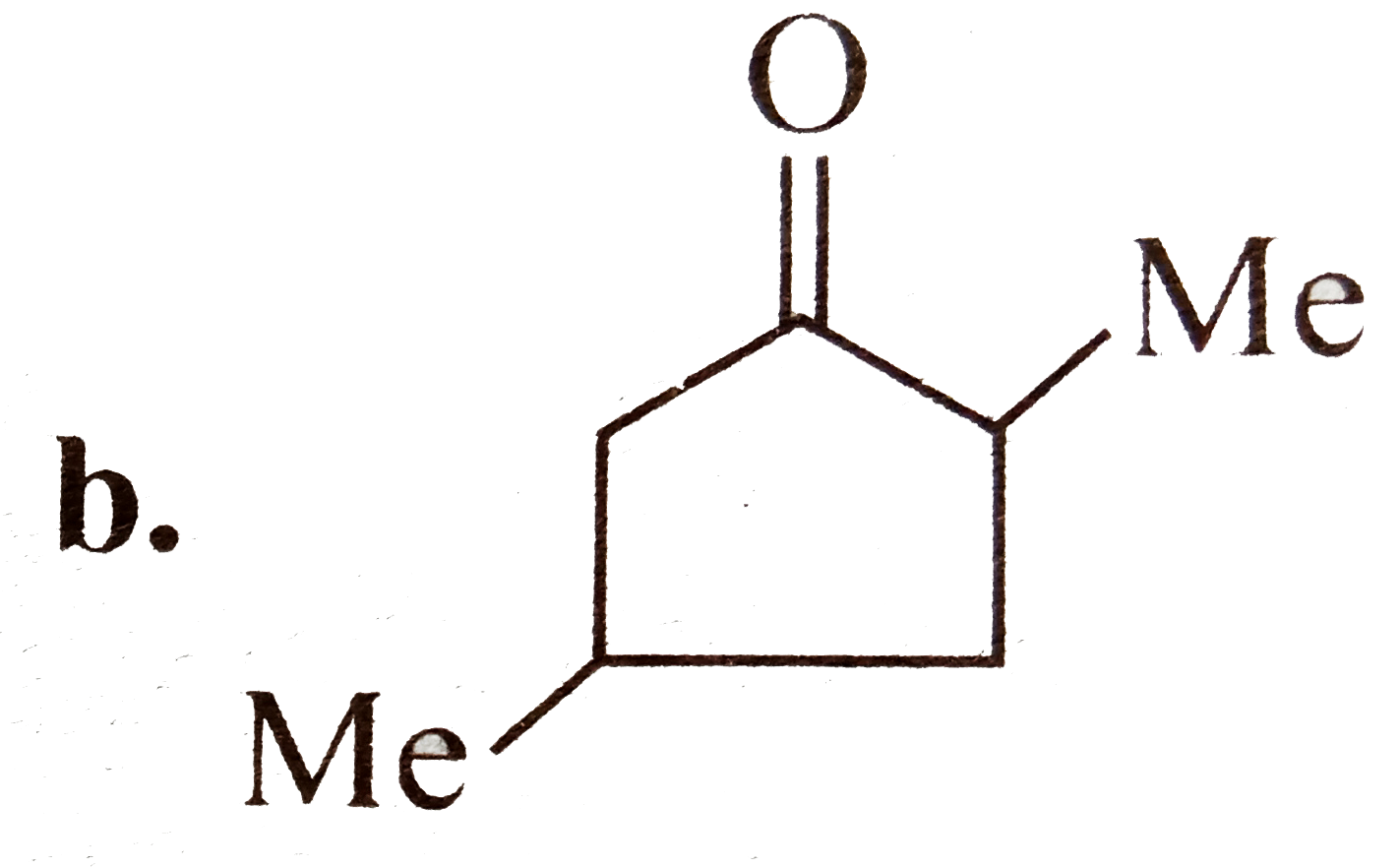A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise True and False|1 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise Fill in the Blanks|1 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise EXERCISES|24 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct Answer|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALKANES AND CYCLOALKANES-Paragraph for problem
- Fifteen milliliters of gaseous hydrocarbo (A) was required for complet...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon (A) was exploded with exce...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon (A) was exploded with exce...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon (A) was exploded with exce...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon (A) was exploded with exce...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon (A) was exploded with exce...
Text Solution
|
- Twenty millilitres of a gaseous hydrocarbon (A) was exploded with exce...
Text Solution
|
- 5 " mL of " a gas A containing only C and H was mixed with an excess o...
Text Solution
|
- Five millilitires of a gas (A) containing only C and H was mixed with ...
Text Solution
|
- 5 " mL of " a gas A containing only C and H was mixed with an excess o...
Text Solution
|
- 5 " mL of " a gas A containing only C and H was mixed with an excess o...
Text Solution
|
- Five millilitires of a gas (A) containing only C and H was mixed with ...
Text Solution
|
- Five millilitires of a gas (A) containing only C and H was mixed with ...
Text Solution
|
- i. Compound (A) ii. Compound (B) iii. Alkene (C) and alkane (D) ...
Text Solution
|
- (i) Compound (A) is:- (ii) Compound (B) is:- (iii) Isomeric hexy...
Text Solution
|
- (i) Compound (A) is:- (ii) Compound (B) is:- (iii) Isomeric hexy...
Text Solution
|
- i. Compound (A) ii. Compound (B) iii. Alkene (C) and alkane (D) ...
Text Solution
|
- A schematic analysis of the reaction of the enantiomer with racemic mi...
Text Solution
|
- A schematic analysis of the reaction of the enantiomer with racemic mi...
Text Solution
|
- A schematic analysis of the reaction of the enantiomer with racemic mi...
Text Solution
|