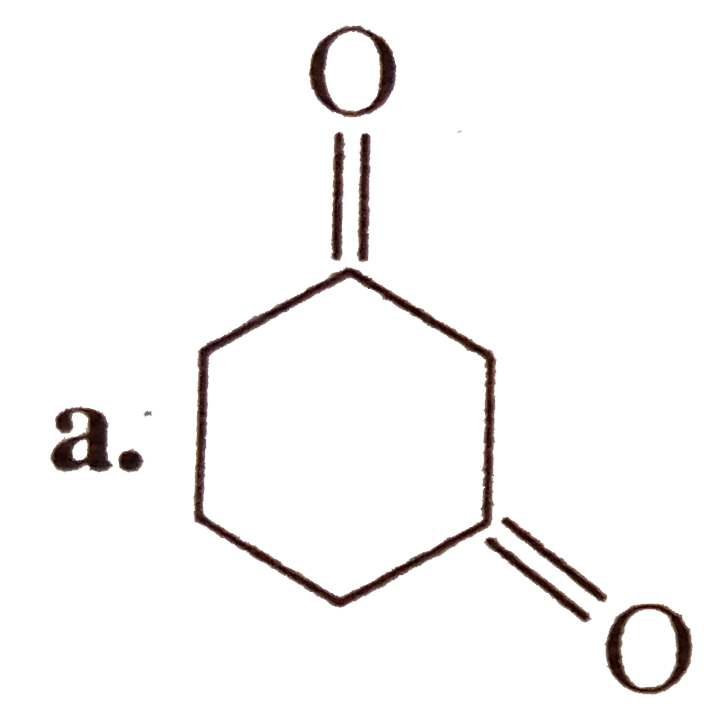
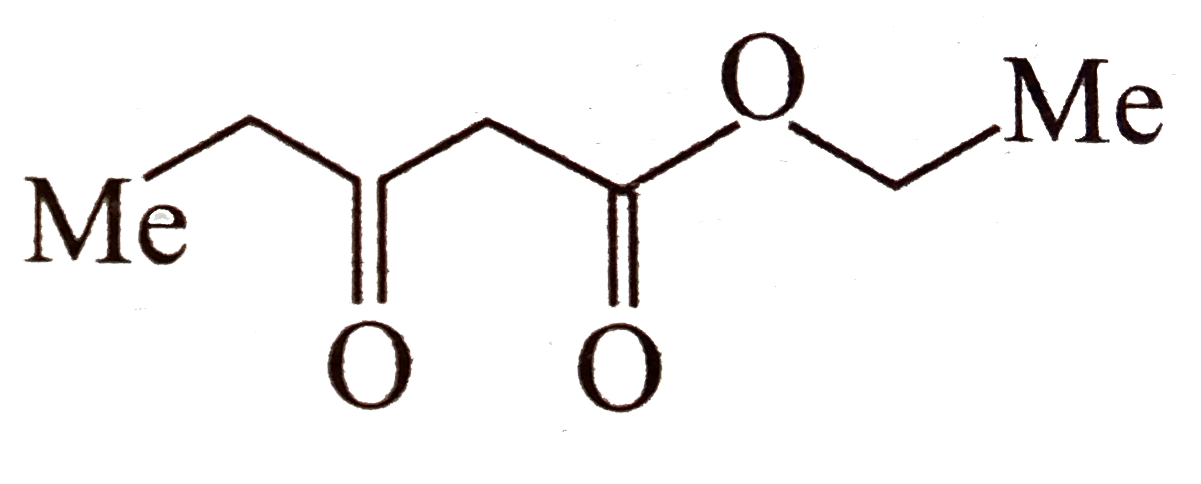
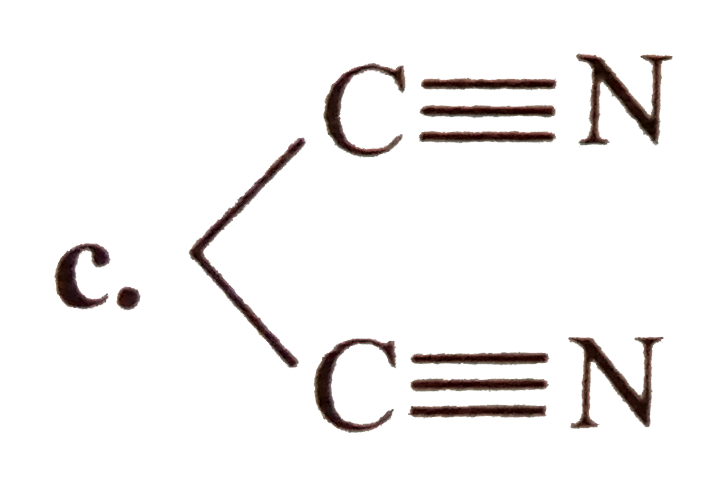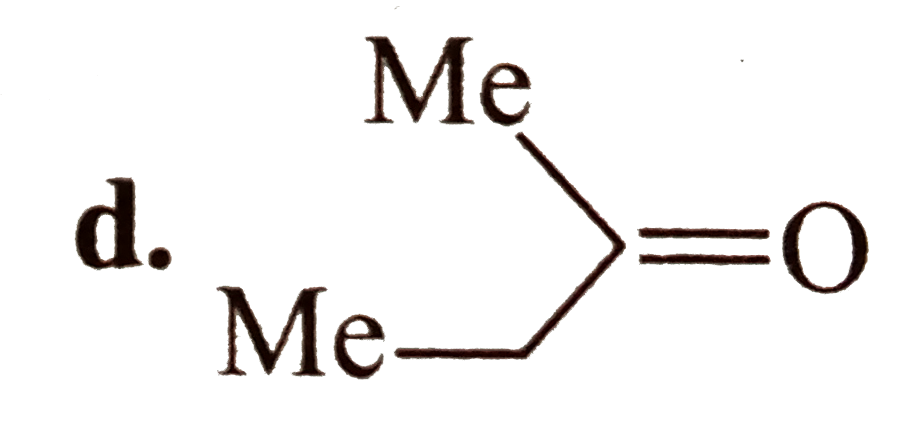A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise Single Correct|45 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives|13 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise Reasoning and Assertion|2 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct Answer|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALKANES AND CYCLOALKANES-MCQ
- An automobile engine fuel has cetane number of 80. Which of the follow...
Text Solution
|
- CH(4) can be prepared by the reaction of H(2)O with:
Text Solution
|
- When aqueous of sodium ethanote is electrolysed, the product(s) at ano...
Text Solution
|
- In the destructive distillation of coal, at 443 - 503 K temperature, a...
Text Solution
|
- Which of the following compounds are isolable ? a.
Text Solution
|
- Which of the following compounds contain active methylene group ?
Text Solution
|
- Write the structure (s) of the simplest alkane (s), with fewest number...
Text Solution
|
- Which of the following statements is/are correct ?
Text Solution
|
- Which of the following statements is/are correct in the synthesis of c...
Text Solution
|
- Which of the following statement (s) about cyclohexane is/are wrong? (...
Text Solution
|
- Which of the following reactions will not give a four-membered cyclic ...
Text Solution
|
- Which of the following statements is/are correct ?
Text Solution
|
- Structure of naturally occurring steroide cholesterol is given: W...
Text Solution
|
- Which of the statements is/are correct about the following reactions ?...
Text Solution
|
- C(6)H(12) (A)overset (Br(2)//hv)underset (Monobromination) rarr One is...
Text Solution
|
- Which statements is/are correct about the conformer of cis and trans 1...
Text Solution
|
- Which of the following statements is/are wrong about pericyclic reacti...
Text Solution
|
- Which statements is/are correct about the following reaction ?
Text Solution
|
- What is the normality of a 1 M solution of H3PO4
Text Solution
|
- Which of the follolwing compounds undergoes easy decarboxylation on he...
Text Solution
|