A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALKANES AND CYCLOALKANES-Single Correct
- There are two ways for the preparation of 2,2-dimethylbutane by Corey...
Text Solution
|
- Which of the statement is/are true about the reactivity of halogenatio...
Text Solution
|
- With the help of the following equation and data choose the wrong stat...
Text Solution
|
Text Solution
|
- Which of the following statements is wrong ?
Text Solution
|
- Arrange the following compounds in the increasing order of homolytic (...
Text Solution
|
- There is no ring strain in cyclohexane, but cyclobutane has an angle s...
Text Solution
|
- Octane number can be changed by:
Text Solution
|
- Which of the following yields both alkane and alkene ?
Text Solution
|
- Hydrocarbon which is liquid at room temperature is
Text Solution
|
- In the free-radical chlorination of methane, the chain-intiation step ...
Text Solution
|
- 2-Methyl butane reacting with Br(2) in sunlight mainly gives:
Text Solution
|
- Which of the following has the lowest boiling point ?
Text Solution
|
- Which hydrocarbnon is mainly present in gobar gas ?
Text Solution
|
- Methyl bromide is converted into ethane by heating it in ether medium ...
Text Solution
|
- Reactivity of hydrogen atoms attached to different carbon atoms in alk...
Text Solution
|
- Of the five isomeric hexanes, the isomer which can give two monochlori...
Text Solution
|
- The compound having only primary hydrogen atoms is
Text Solution
|
- Which of the following has the higest knocking property ?
Text Solution
|
- Which of the following reactions will not give propane ?
Text Solution
|
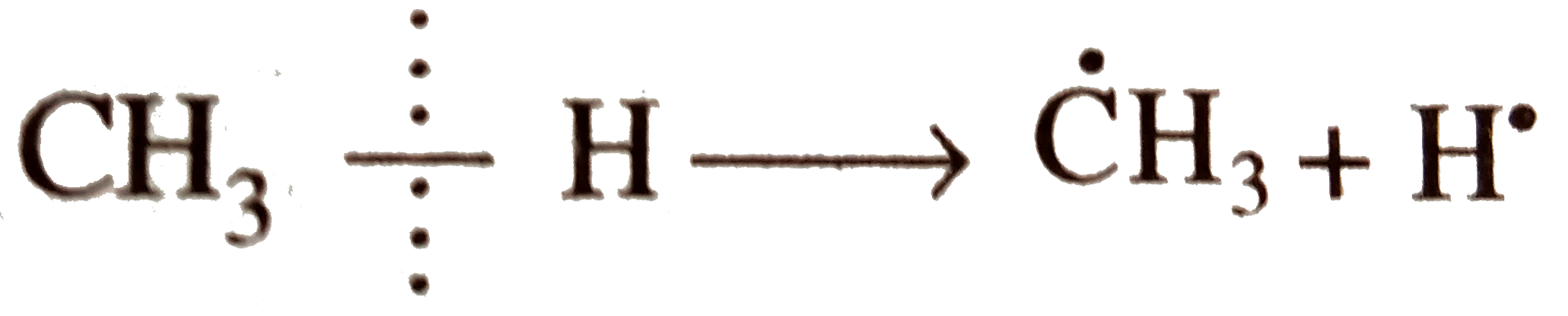 `E_(act) = + 435.1 kJ`
`E_(act) = + 435.1 kJ` 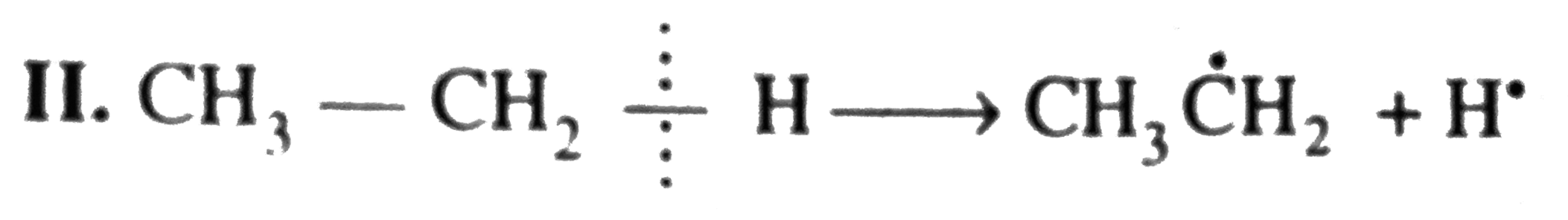 `E_(act) = + 410.0 kJ`
`E_(act) = + 410.0 kJ` 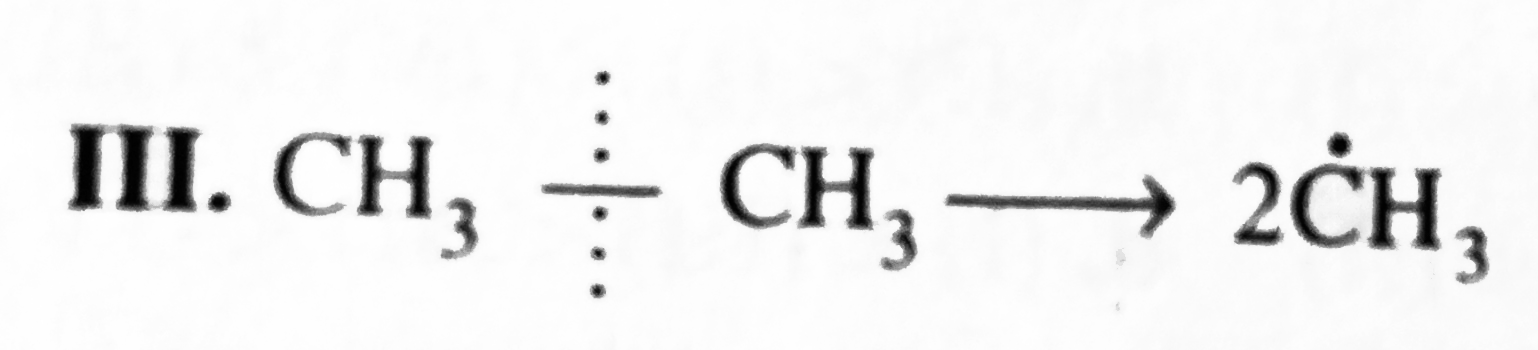 `E_(act) = + 368.0 kJ`
`E_(act) = + 368.0 kJ` 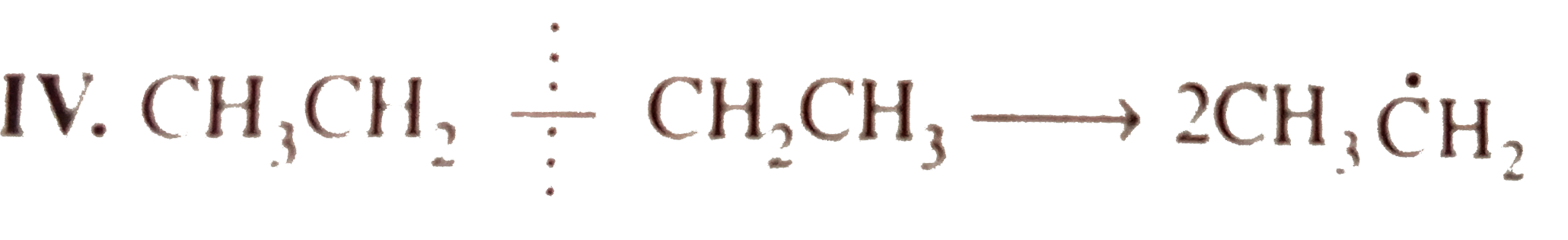 `E_(act) = + 343.0 kJ`
`E_(act) = + 343.0 kJ` 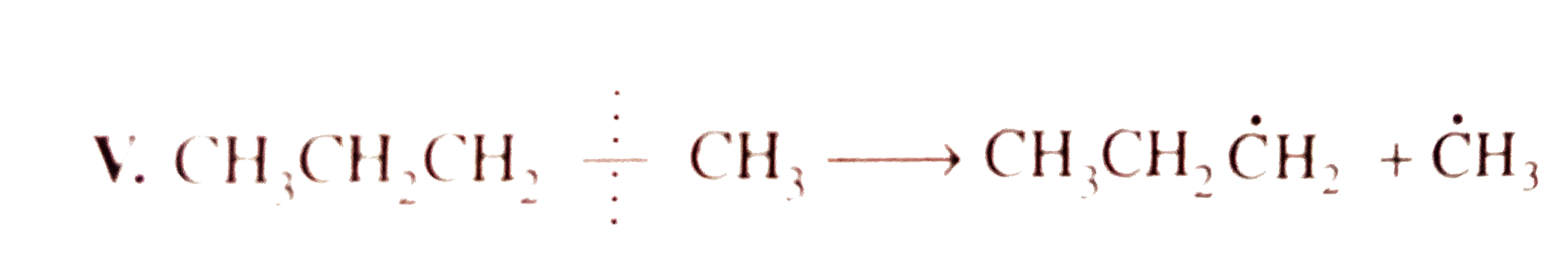 `E_(act) = 355.6 kJ`
`E_(act) = 355.6 kJ`