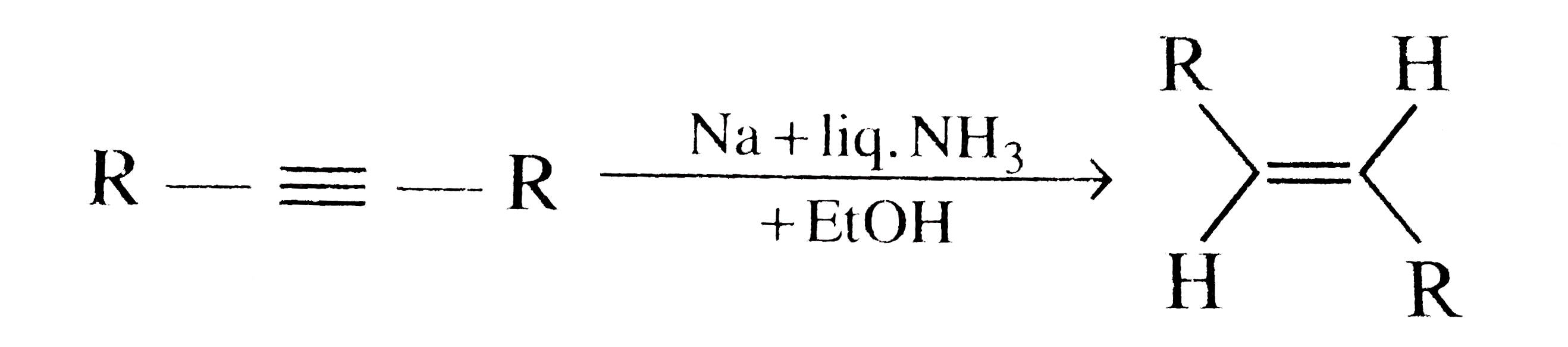A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALKYNES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Archives - Single Correct Answer Type)|6 VideosALKYNES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Archives - Fill in the Blanks Type)|3 VideosALKYNES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct Answers Type)|25 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct Answer|14 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY ENGLISH|Exercise chapter-7 Single correct answer|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALKYNES-Exercises (Single Correct Answers Type)
- Which of the following is propargyl group?
Text Solution
|
- What is the smallest ring that can accommodate a triple bond?
Text Solution
|
- In the conversion of alkyne to trans-alkene by Birch reduction using a...
Text Solution
|
- In the reaction, R--=-Roverset(Birch)underset(reduction)rarr source ...
Text Solution
|
- In the reaction:
Text Solution
|
- Interconversion of terminal to internal alkyne and vice versa takes pl...
Text Solution
|
- R-underset((A))-=--=-Roverset("Lindlar's catalyst" +H2)rarr(B) Co...
Text Solution
|
- Compounds (B) and (C) are:
Text Solution
|
- (B), (C), and (D), respectively, are
Text Solution
|
- Expand the following condensed formulas into their complete structural...
Text Solution
|
Text Solution
|
Text Solution
|
- A, B, and C:
Text Solution
|
- Compound (X) on complete catalytic hydrogenation with H2//Pt gives an ...
Text Solution
|
- Cl3-underset((X))-=-Hunderset(D2+P-2catalyst)rarr(A)overset(HCl)rarr(B...
Text Solution
|
- H--=-Hoverset((i)O3)underset((ii)(H2O)//(Zn))rarr(A)overset(Zn//CH3COO...
Text Solution
|
- Which one of the following does not dissolve in conc. H2SO4?
Text Solution
|
- 2H--=-Hunderset(NH4Cl)overset(CuCl)rarrAoverset(H2+Ni2B)rarrBunderset(...
Text Solution
|
- 2HC-=CHoverset(CH2Cl2)underset(+NH4Cl)rarr(A)overset(1 mol)underset(HC...
Text Solution
|
- 2HC-=Choverset(Cu^(2+)+O2)rarr(A)overset(1 mol)underset(HCl)rarr(B) ...
Text Solution
|