Text Solution
Verified by Experts
Topper's Solved these Questions
GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|15 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Subjective )|17 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Nuclear Chemistry (NCERT Exercise)|29 Videos
Similar Questions
Explore conceptually related problems
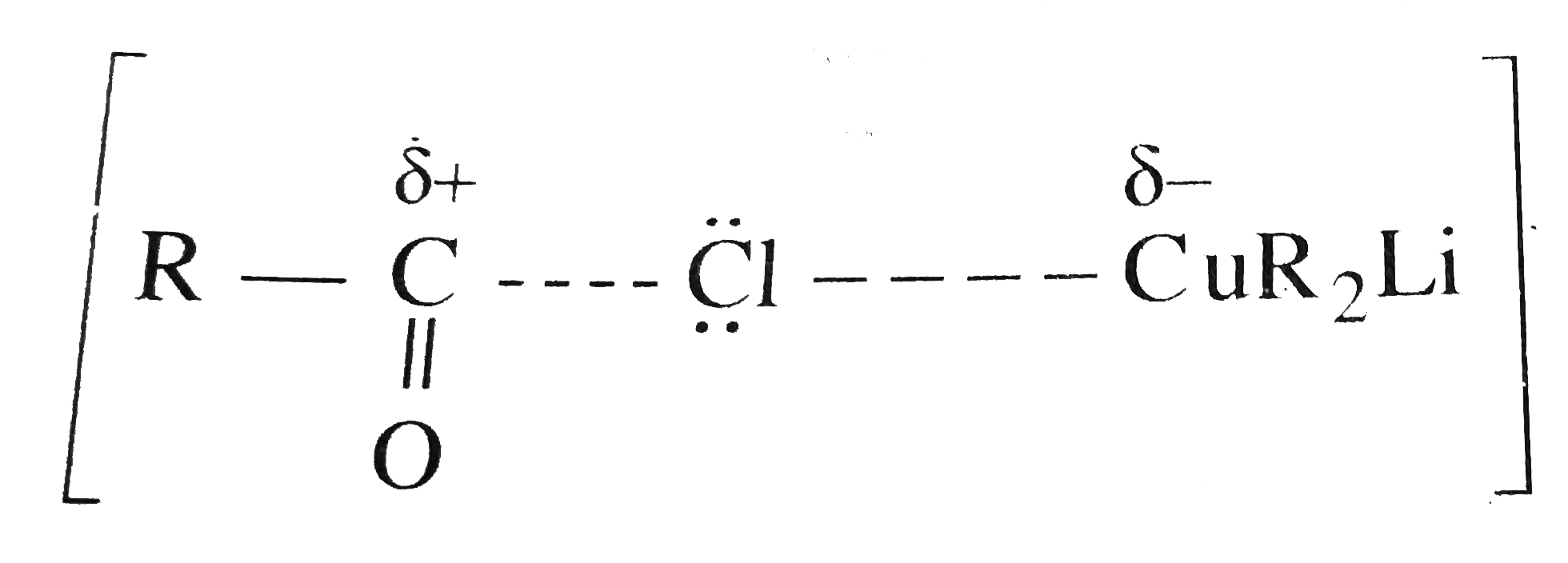
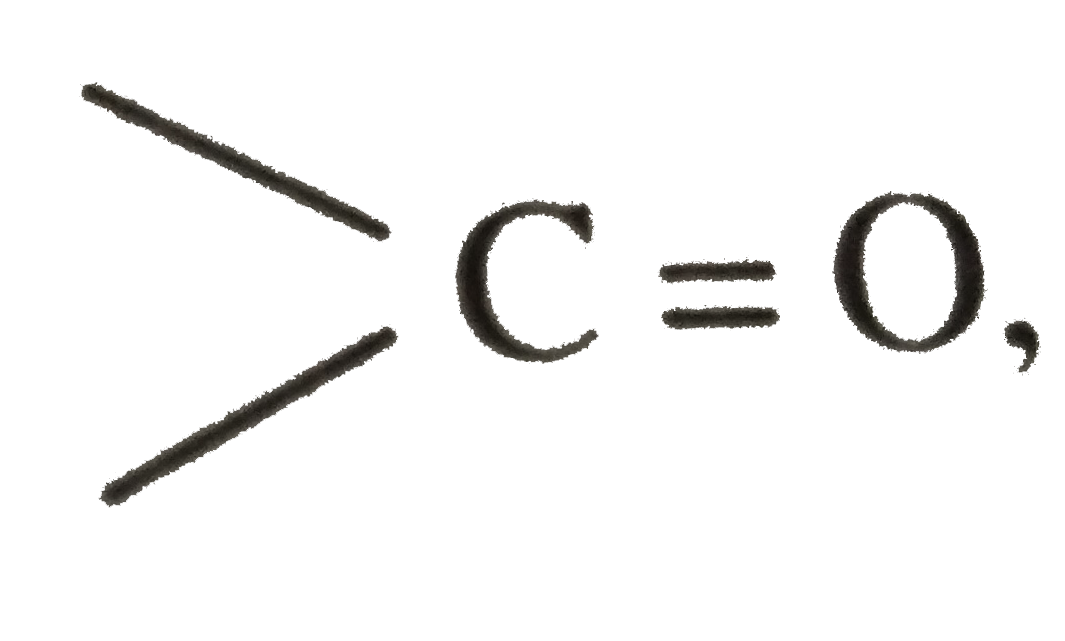 forming an acylium-like ion with greater `+ delta` charge on the carbon. This strong electrophilic ion can form a bond even with the weak nucleophlic `R` of `R_2 CuLi` or `R_2 Cd`.
forming an acylium-like ion with greater `+ delta` charge on the carbon. This strong electrophilic ion can form a bond even with the weak nucleophlic `R` of `R_2 CuLi` or `R_2 Cd`.