Concrete is produced form a mixture of cement, water and small stones. Small amount of gypsum, `CaSO_(4).2H_(2)O` is added in coment production to impove the subsequent hardening of concrete. The elevated temperature during the production of cement may lead to the formation of unwanted hemihydrate `CaSO_(4)(1)/(2)H_(2)O` according to reaction.
`CaSO_(4)2H_(2)O(s) rarr CaSO_(4)(1)/(2)H_(2)O(s)+(3)/(2)H_(2)O(g)`
The `Delta_(f)H^(Theta) of CaSO_(2).2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s),H_(2)O(g)`
are `-2021.0 kJ mol^(-1), -1575.0 kJ mol^(-1)` and `-241.8 kJ mol^(-1)`, respectively. The respective values of their standard entropies are `194.0, 130.0` and `188.0 J K^(-1)mol^(-1)`. The values of `R = 8.314JK^(-1)mol^(-1) = 0.0831L` bar `mol^(-1)K^(-1)`.
Answer the following questions on the basis of above information.
The equilibrium pressure of water vapour in closed vessel containing `CaSO_(4)2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s)` and `H_(2)O(g)` at `298K` (Antilog `-3.14 = 7.24 xx 10^(-4))` is
Concrete is produced form a mixture of cement, water and small stones. Small amount of gypsum, `CaSO_(4).2H_(2)O` is added in coment production to impove the subsequent hardening of concrete. The elevated temperature during the production of cement may lead to the formation of unwanted hemihydrate `CaSO_(4)(1)/(2)H_(2)O` according to reaction.
`CaSO_(4)2H_(2)O(s) rarr CaSO_(4)(1)/(2)H_(2)O(s)+(3)/(2)H_(2)O(g)`
The `Delta_(f)H^(Theta) of CaSO_(2).2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s),H_(2)O(g)`
are `-2021.0 kJ mol^(-1), -1575.0 kJ mol^(-1)` and `-241.8 kJ mol^(-1)`, respectively. The respective values of their standard entropies are `194.0, 130.0` and `188.0 J K^(-1)mol^(-1)`. The values of `R = 8.314JK^(-1)mol^(-1) = 0.0831L` bar `mol^(-1)K^(-1)`.
Answer the following questions on the basis of above information.
The equilibrium pressure of water vapour in closed vessel containing `CaSO_(4)2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s)` and `H_(2)O(g)` at `298K` (Antilog `-3.14 = 7.24 xx 10^(-4))` is
`CaSO_(4)2H_(2)O(s) rarr CaSO_(4)(1)/(2)H_(2)O(s)+(3)/(2)H_(2)O(g)`
The `Delta_(f)H^(Theta) of CaSO_(2).2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s),H_(2)O(g)`
are `-2021.0 kJ mol^(-1), -1575.0 kJ mol^(-1)` and `-241.8 kJ mol^(-1)`, respectively. The respective values of their standard entropies are `194.0, 130.0` and `188.0 J K^(-1)mol^(-1)`. The values of `R = 8.314JK^(-1)mol^(-1) = 0.0831L` bar `mol^(-1)K^(-1)`.
Answer the following questions on the basis of above information.
The equilibrium pressure of water vapour in closed vessel containing `CaSO_(4)2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s)` and `H_(2)O(g)` at `298K` (Antilog `-3.14 = 7.24 xx 10^(-4))` is
Text Solution
Verified by Experts
(a) `underset("Benzoyl chloride")(2Ph-overset(O)overset(||)(C)-Cl)+underset("cadmium")underset("Dimethyl")(Me_(2)Cd)tounderset((A))(2Ph)-underset("Acetophenone")underset((B))(overset(O)overset(||)(C))-Me+underset("choloride")underset("Cadmium")underset((C))(CdCl_(2))`
(b) `(D)` is a cyclic ester, called lactone. The ester reacts with `2 mol` of `G.R` to give `3^@` alcohol. (Two `R` groups come from the `G.R`)
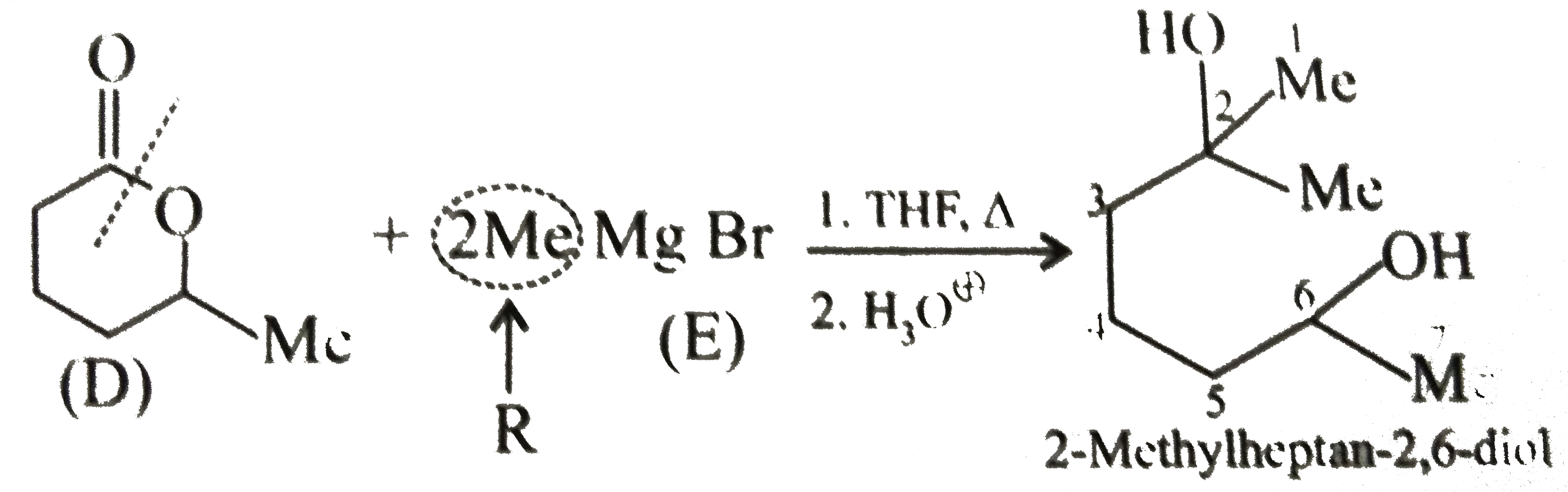
( c) `(G)` is a cyclic anhydride. The anhydride reacts with `2 mol` of `G.R` to give `2^@` alcohol (two `R` groups come from the `G.R`) Nucleophilic addition `(NA)` reaction is favoured by the electron-withdrawing group `(EWG)` and disfavoured by electron-donating group `(EDG)`. The cleavage of ring takes place from the side not containing the `EDG` (Me group).
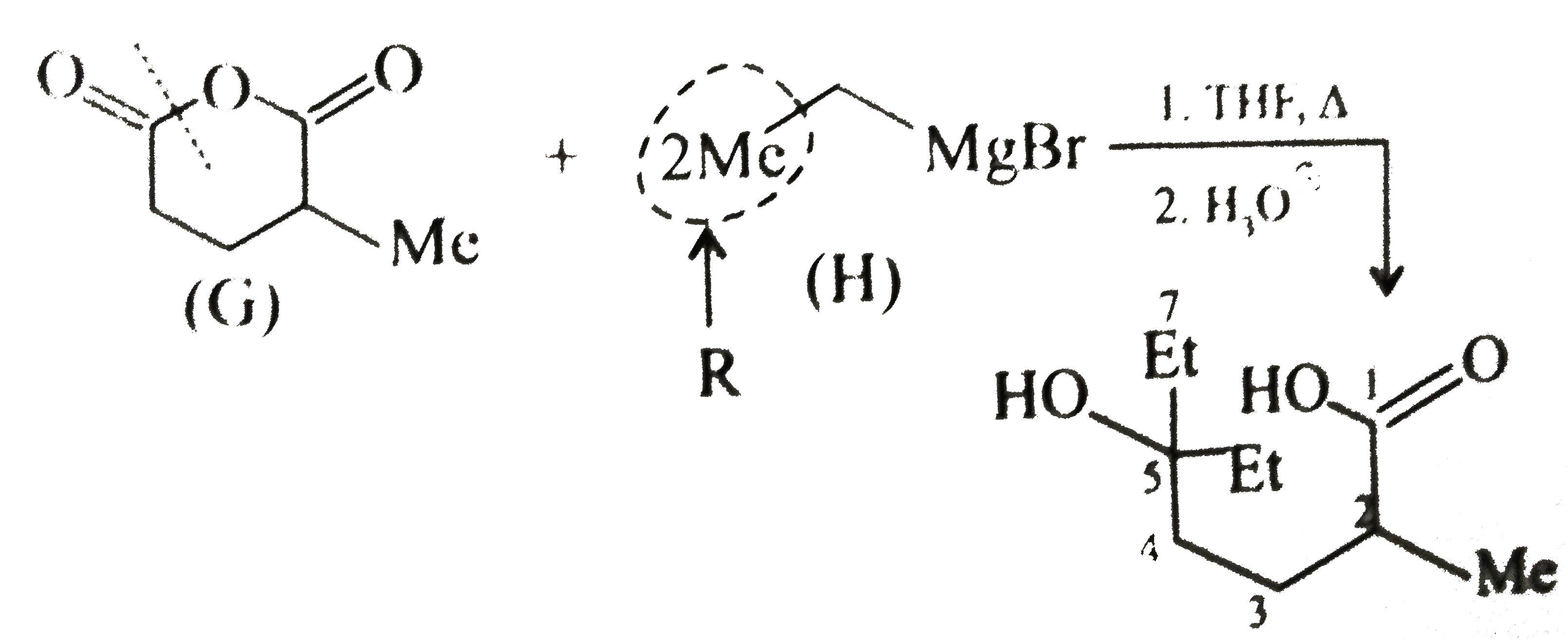
(d) `(J)` is a cyclic amide, called lactam. The amide reacts with `2 mol` of `G.R` to give ketone (one `R` comes from the `G.R`).

In `(e),(f)` and `(g)`, less reactive `R_2 CuLi` (dialkyl lithium cuprate) reacts only with more reactive `(-overset(O)overset(||)(C)-Cl)`
group, but more reactive `G.R` reacts with both `(-overset(O)overset(||)(C)-Cl)` and `(-C -= N)` groups. Two moles of `G.R` reacts with `(-overset(O)overset(||)(C)-Cl)` group to give `3^@` alcohol (two `R` groups come from the `G.R`) , but with `(-C -= N)` group, the `G.R` gives ketone (one `R` group comes from `G.R`).
(e)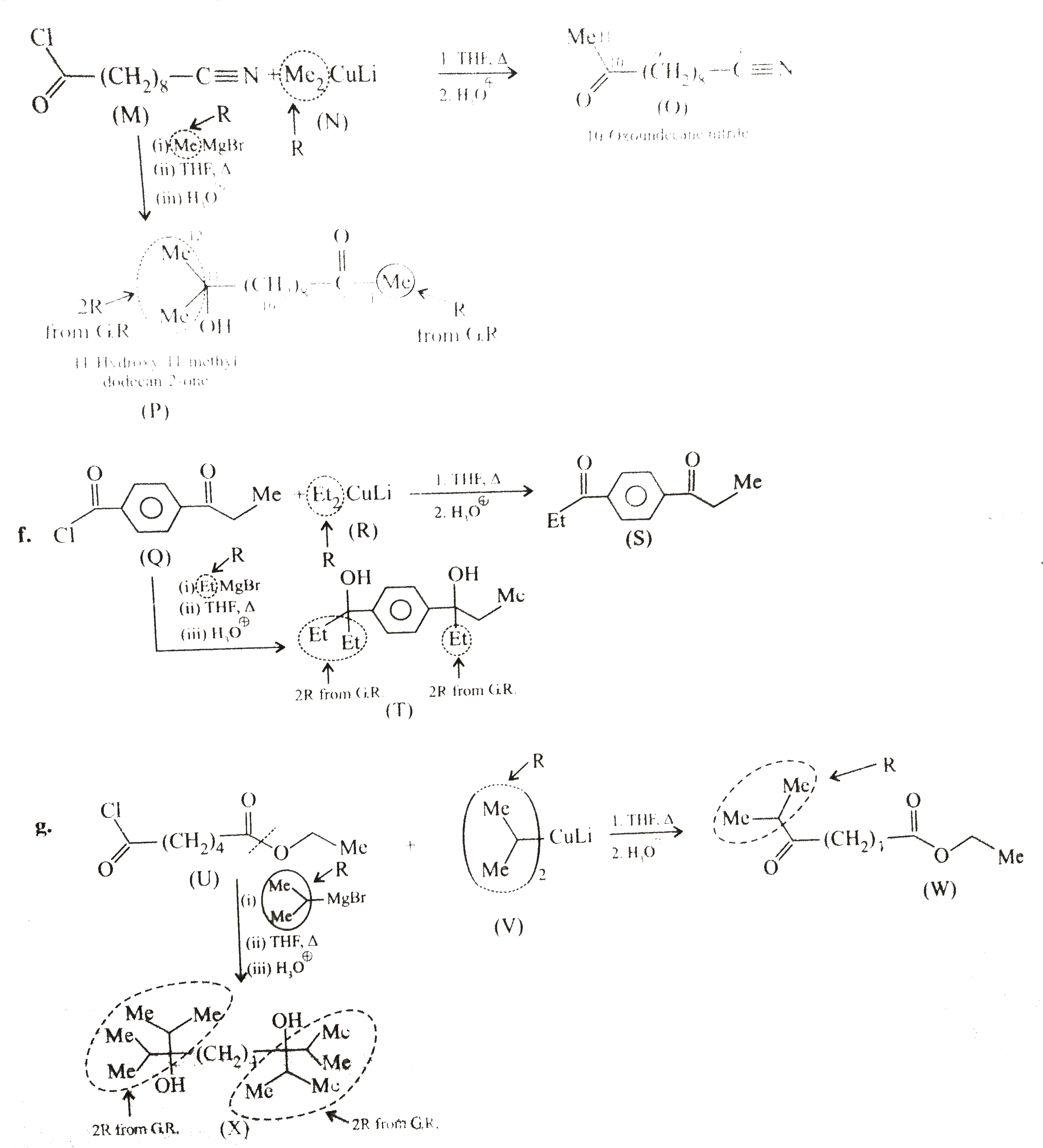
(b) `(D)` is a cyclic ester, called lactone. The ester reacts with `2 mol` of `G.R` to give `3^@` alcohol. (Two `R` groups come from the `G.R`)
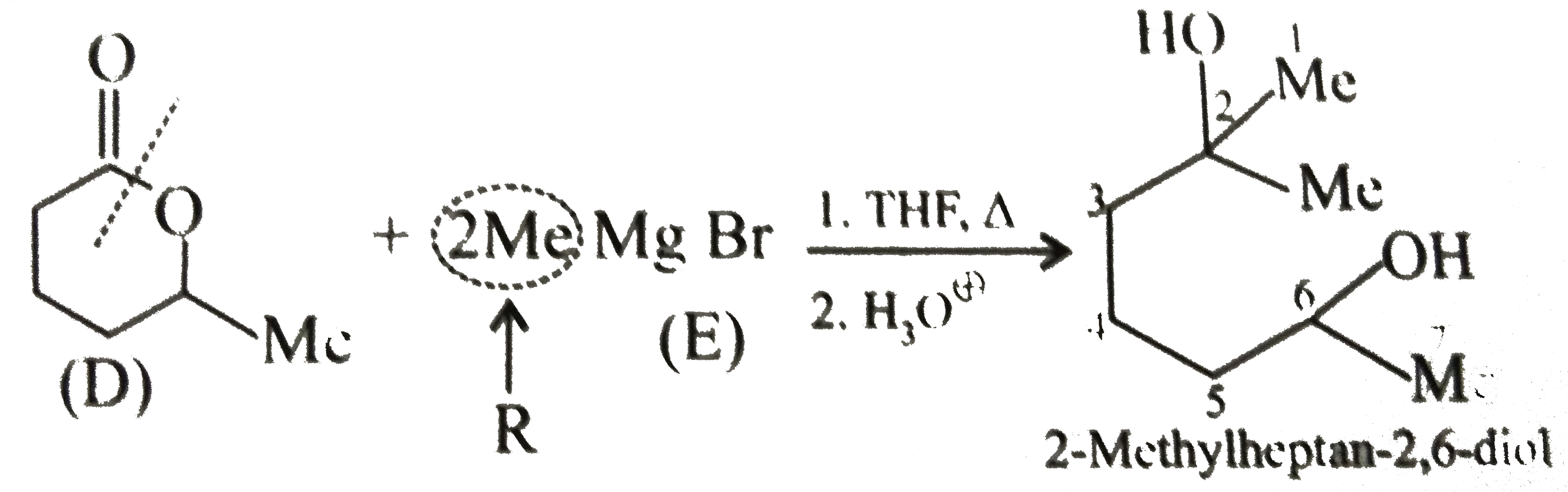
( c) `(G)` is a cyclic anhydride. The anhydride reacts with `2 mol` of `G.R` to give `2^@` alcohol (two `R` groups come from the `G.R`) Nucleophilic addition `(NA)` reaction is favoured by the electron-withdrawing group `(EWG)` and disfavoured by electron-donating group `(EDG)`. The cleavage of ring takes place from the side not containing the `EDG` (Me group).
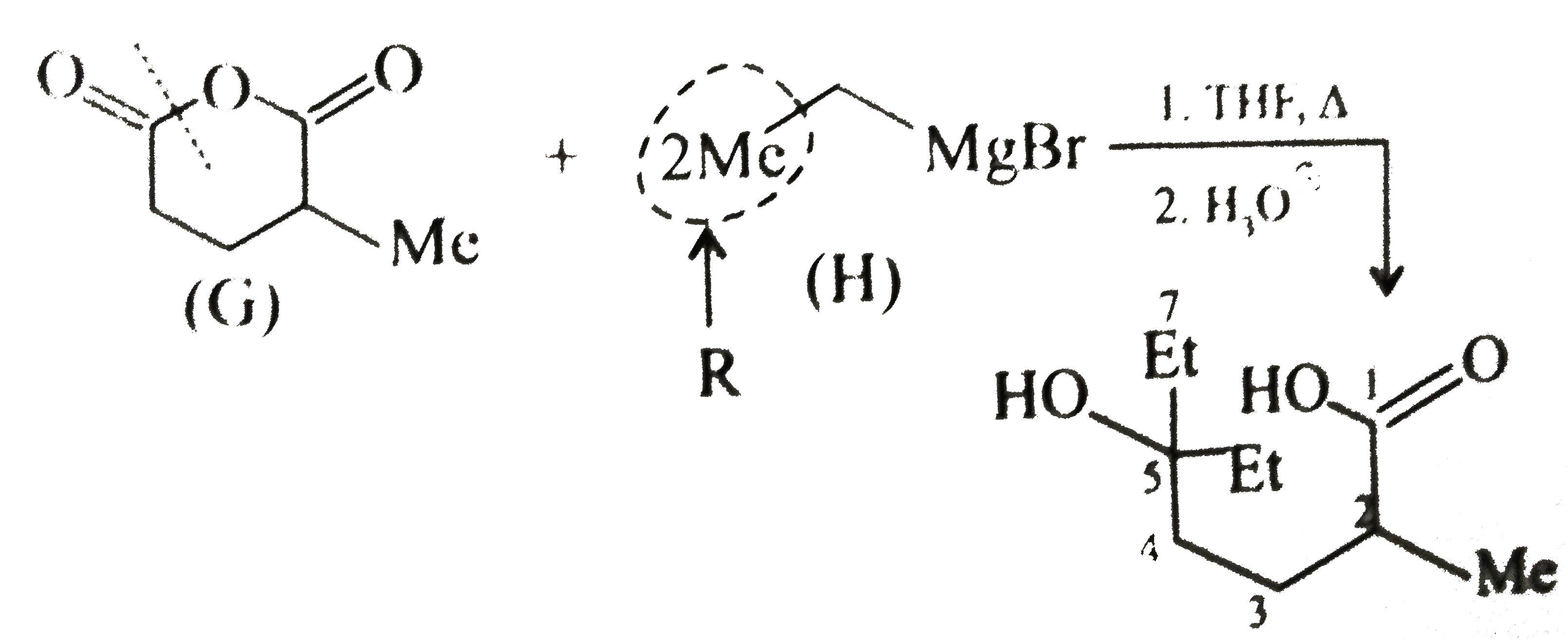
(d) `(J)` is a cyclic amide, called lactam. The amide reacts with `2 mol` of `G.R` to give ketone (one `R` comes from the `G.R`).

In `(e),(f)` and `(g)`, less reactive `R_2 CuLi` (dialkyl lithium cuprate) reacts only with more reactive `(-overset(O)overset(||)(C)-Cl)`
group, but more reactive `G.R` reacts with both `(-overset(O)overset(||)(C)-Cl)` and `(-C -= N)` groups. Two moles of `G.R` reacts with `(-overset(O)overset(||)(C)-Cl)` group to give `3^@` alcohol (two `R` groups come from the `G.R`) , but with `(-C -= N)` group, the `G.R` gives ketone (one `R` group comes from `G.R`).
(e)

Topper's Solved these Questions
GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|15 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Subjective )|17 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Nuclear Chemistry (NCERT Exercise)|29 Videos
Similar Questions
Explore conceptually related problems
Concrete is produced form a mixture of cement, water and small stones. Small amount of gypsum, CaSO_(4).2H_(2)O is added in coment production to impove the subsequent hardening of concrete. The elevated temperature during the production of cement may lead to the formation of unwanted hemihydrate CaSO_(4)(1)/(2)H_(2)O according to reaction. CaSO_(4)2H_(2)O(s) rarr CaSO_(4)(1)/(2)H_(2)O(s)+(3)/(2)H_(2)O(g) The Delta_(f)H^(Theta) of CaSO_(2).2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s),H_(2)O(g) are -2021.0 kJ mol^(-1), -1575.0 kJ mol^(-1) and -241.8 kJ mol^(-1) , respectively. The respective values of their standard entropies are 194.0, 130.0 and 188.0 J K^(-1)mol^(-1) at 298K. The values of R = 8.314JK^(-1)mol^(-1) = 0.0831L bar mol^(-1)K^(-1) . Answer the following questions on the basis of above information. The value of equilibrium for reaction is
Concrete is produced form a mixture of cement, water and small stones. Small amount of gypsum, CaSO_(4).2H_(2)O is added in coment production to impove the subsequent hardening of concrete. The elevated temperature during the production of cement may lead to the formation of unwanted hemihydrate CaSO_(4)(1)/(2)H_(2)O according to reaction. CaSO_(4)2H_(2)O(s) rarr CaSO_(4)(1)/(2)H_(2)O(s)+(3)/(2)H_(2)O(g) The Delta_(f)H^(Theta) of CaSO_(2).2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s),H_(2)O(g) are -2021.0 kJ mol^(-1), -1575.0 kJ mol^(-1) and -241.8 kJ mol^(-1) , respectively. The respective values of their standard entropies are 194.0, 130.0 and 188.0 J K^(-1)mol^(-1) . The values of R = 8.314JK^(-1)mol^(-1) = 0.0831L bar mol^(-1)K^(-1) . Answer the following questions on the basis of above information. The formation of CaSO_(4)(1)/(2)H_(2)O at 298K is
Concrete is produced form a mixture of cement, water and small stones. Small amount of gypsum, CaSO_(4).2H_(2)O is added in coment production to impove the subsequent hardening of concrete. The elevated temperature during the production of cement may lead to the formation of unwanted hemihydrate CaSO_(4)(1)/(2)H_(2)O according to reaction. CaSO_(4)2H_(2)O(s) rarr CaSO_(4)(1)/(2)H_(2)O(s)+(3)/(2)H_(2)O(g) The Delta_(f)H^(Theta) of CaSO_(2).2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s),H_(2)O(g) are -2021.0 kJ mol^(-1), -1575.0 kJ mol^(-1) and -241.8 kJ mol^(-1) , respectively. The respective values of their standard entropies are 194.0, 130.0 and 188.0 J K^(-1)mol^(-1) . The values of R = 8.314JK^(-1)mol^(-1) = 0.0831L bar mol^(-1)K^(-1) . Answer the following questions on the basis of above information. The value of DeltaG^(Theta) for the reaction at 298K is
Concrete is produced form a mixture of cement, water and small stones. Small amount of gypsum, CaSO_(4).2H_(2)O is added in cement production to improve the subsequent hardening of concrete. The elevated temperature during the production of cement may lead to the formation of unwanted hemihydrate CaSO_(4)(1)/(2)H_(2)O according to reaction. CaSO_(4)2H_(2)O(s) rarr CaSO_(4)(1)/(2)H_(2)O(s)+(3)/(2)H_(2)O(g) The Delta_(f)H^(Theta) of CaSO_(2).2H_(2)O(s),CaSO_(4)(1)/(2)H_(2)O(s),H_(2)O(g) are -2021.0 kJ mol^(-1), -1575.0 kJ mol^(-1) and -241.8 kJ mol^(-1) , respectively. The respective values of their standard entropies are 194.0, 130.0 and 188.0 J K^(-1)mol^(-1) . The values of R = 8.314JK^(-1)mol^(-1) = 0.0831L bar mol^(-1)K^(-1) . Answer the following questions on the basis of above information. Heat change occurring during conversion of 1kg of CaSO_(4).2H_(2)O(s) (molar mass 172g mol^(-1)) of CaSO_(4)(1)/(2)H_(2)O(s) is equal to
CaSO_(3)darr+H_(2)SO_(4)toCaSO_(4)+SO_(2)uarr+H_(2)O
CaSO_(3)darr+H_(2)SO_(4)toCaSO_(4)+SO_(2)uarr+H_(2)O
In the reaction. H_(2)S+H_(2)O_(2) rarr S+2H_(2)O describes
The equilibrium constant for the reaction CaSO_(4).H_(2)O(s)hArr CaSO_(4).3H_(2)O(s)+2H_(2)O(g) is equal to
Ca(OH)_(2)+SO_(2) to CaSO_(3)darr+H_(2)O
Ca(OH)_(2)+SO_(2) to CaSO_(3)darr+H_(2)O