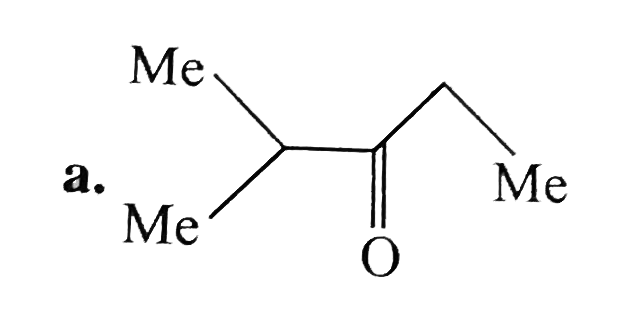
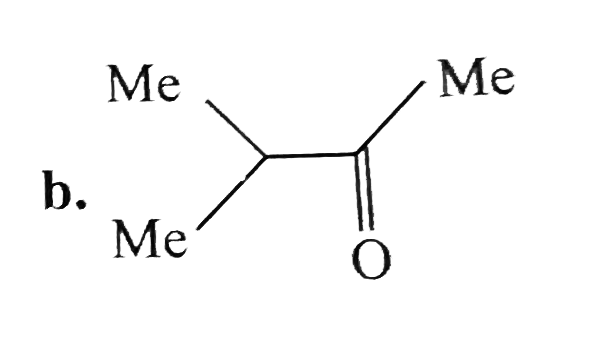
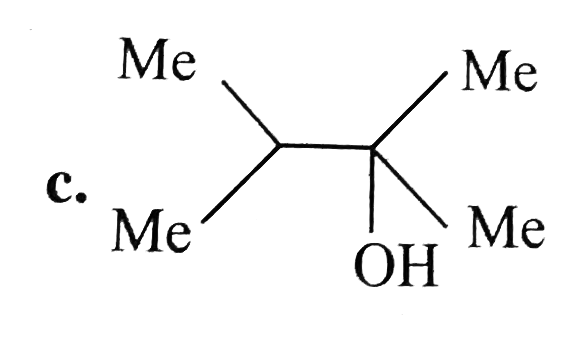
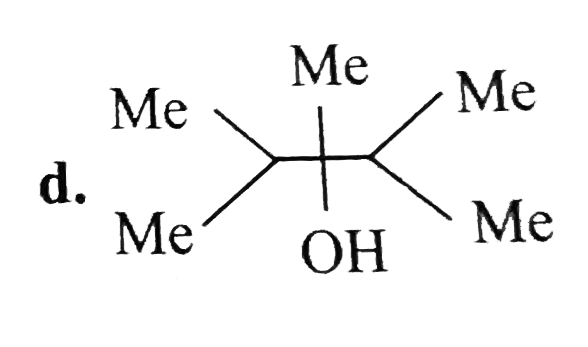
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Single Correct)|4 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (True/False)|1 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct)|16 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Nuclear Chemistry (NCERT Exercise)|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS-Exercises (Single Correct)
- A long solenoid having n = 200 turns per metre has a circular cross-se...
Text Solution
|
- When ethyl ethanoate is treated with excess of MeMgBr, followed by hyd...
Text Solution
|
- When di-isopropyl cadmium is treated with ethanoyl chloride, the produ...
Text Solution
|
- When is treated with C2H5Mg Br, followed by hydrolysis, the product i...
Text Solution
|
- When ethanamide is treated with EtMgBr, followed by hydrolysis, the pr...
Text Solution
|
- Propane is not formed when C3 H7 MgBr is treated with
Text Solution
|
- Alcohol is not formed when RMgX is treated with
Text Solution
|
- . The product is :
Text Solution
|
- The end product ( C) of the following sequence of reaction is : CH -...
Text Solution
|
- . What is A?
Text Solution
|
- overset(|HgSO4//H2 SO4)rarr(A) overset (2 mol of MeMgBr//H3 O^(oplus))...
Text Solution
|
- .
Text Solution
|
- When is treated with followed by hydrolysis, the product is :
Text Solution
|
- The product A is :
Text Solution
|
- (A) and (B) are :
Text Solution
|
- .
Text Solution
|
- .
Text Solution
|
- .
Text Solution
|
- .
Text Solution
|
- .
Text Solution
|