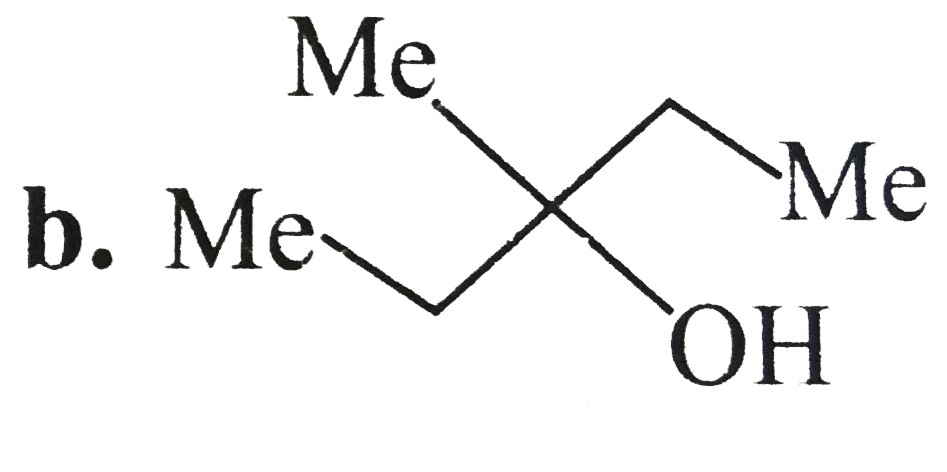
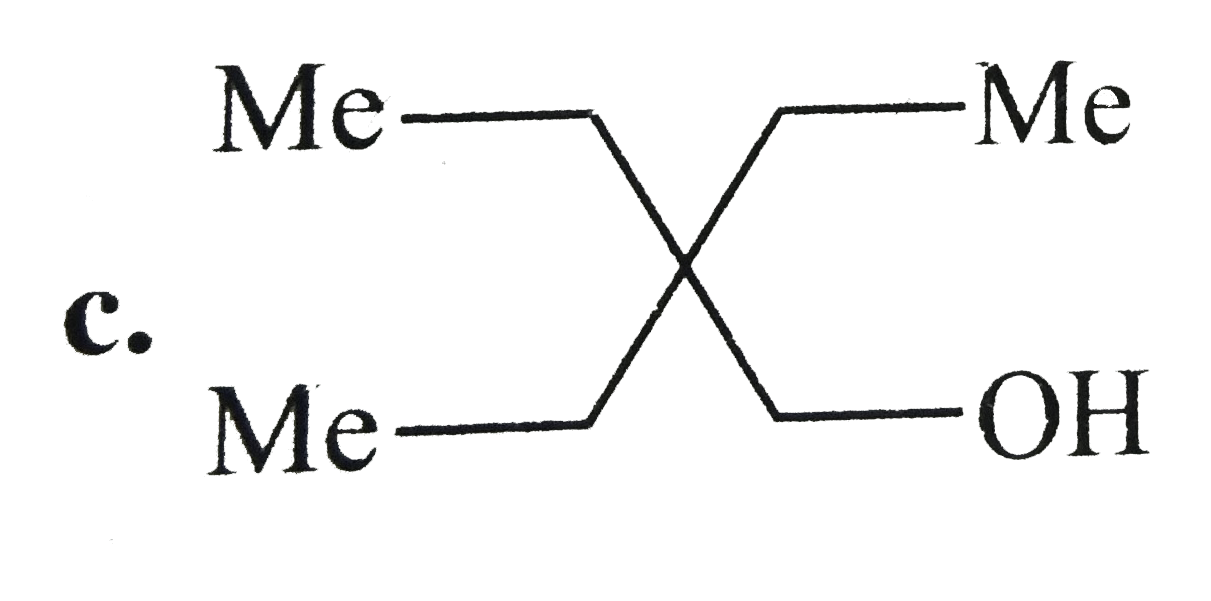
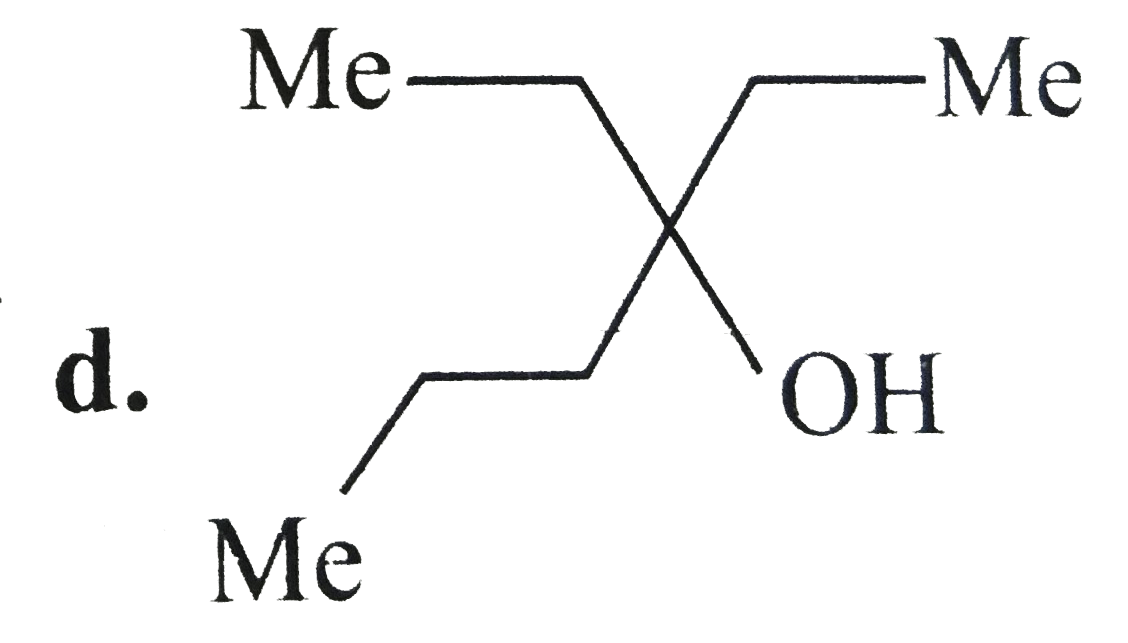
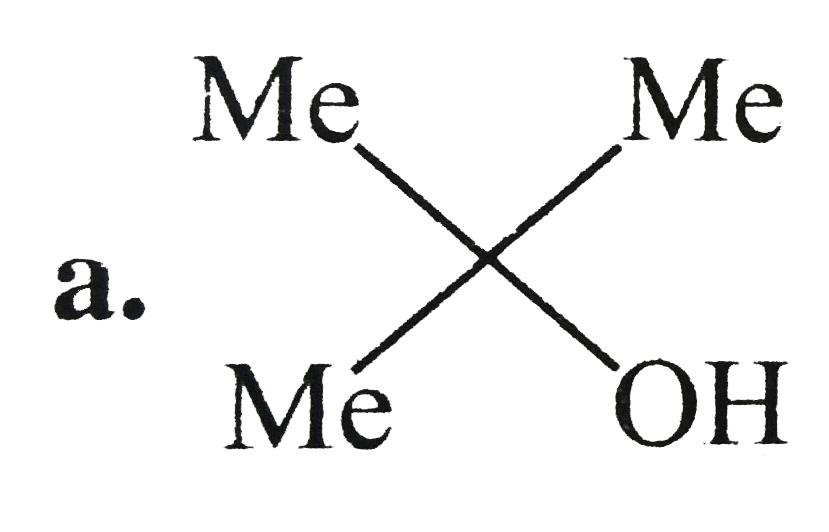A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (True/False)|1 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|9 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct)|47 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Nuclear Chemistry (NCERT Exercise)|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS-Exercises Archives (Single Correct)
- Me3 C-MgCl on reaction with D2 O produces :
Text Solution
|
- Ethyl ester overset(MeMgBr) underset(excess)rarr P. The product P will...
Text Solution
|
- The order of reactivity of phenyl magnesium bromide with the following...
Text Solution
|
- When phenyl magnesium bormide reacts with t-butanol the product would ...
Text Solution
|