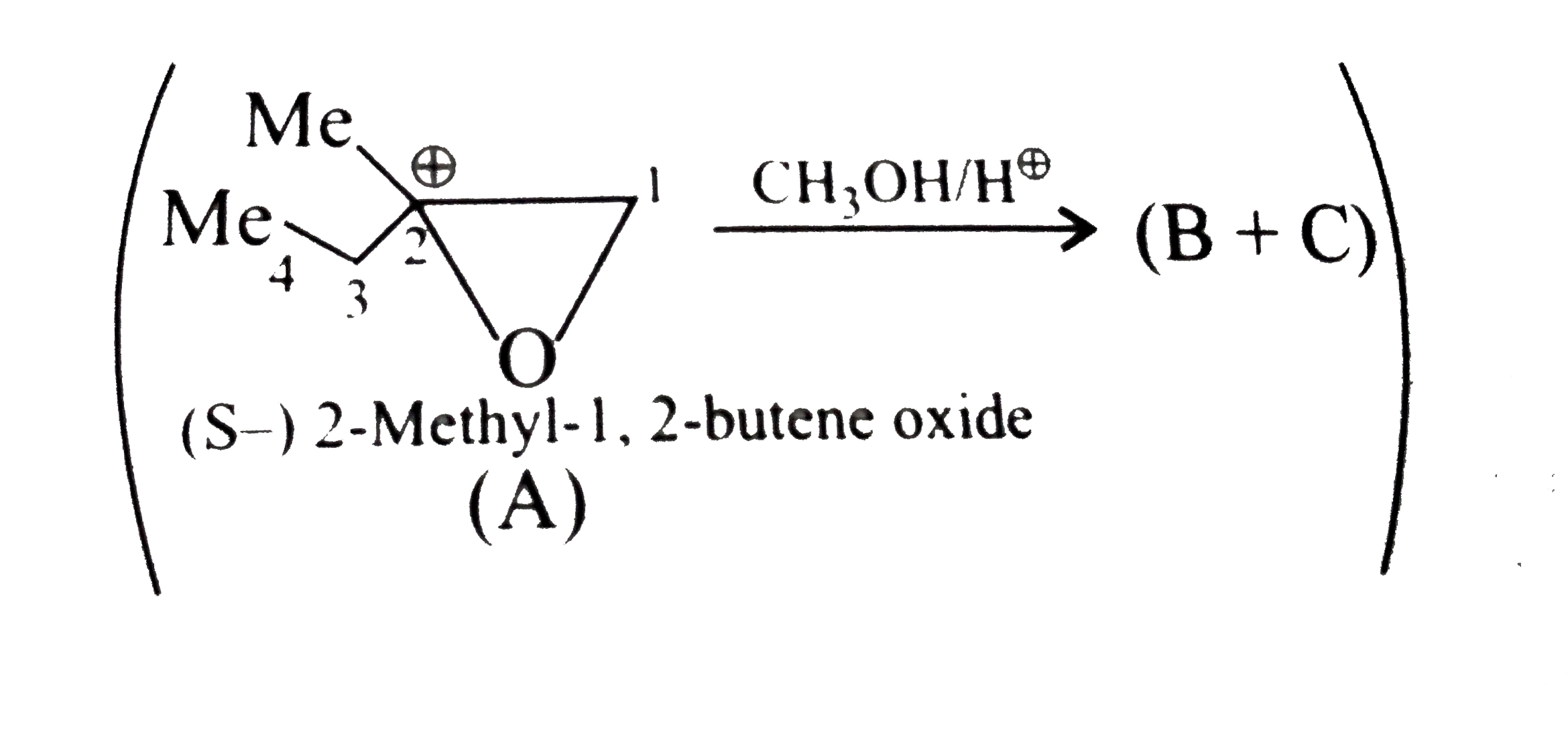Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALCOHOL,PHENOL AND ETHERS-Archives Analytical And Descriptive
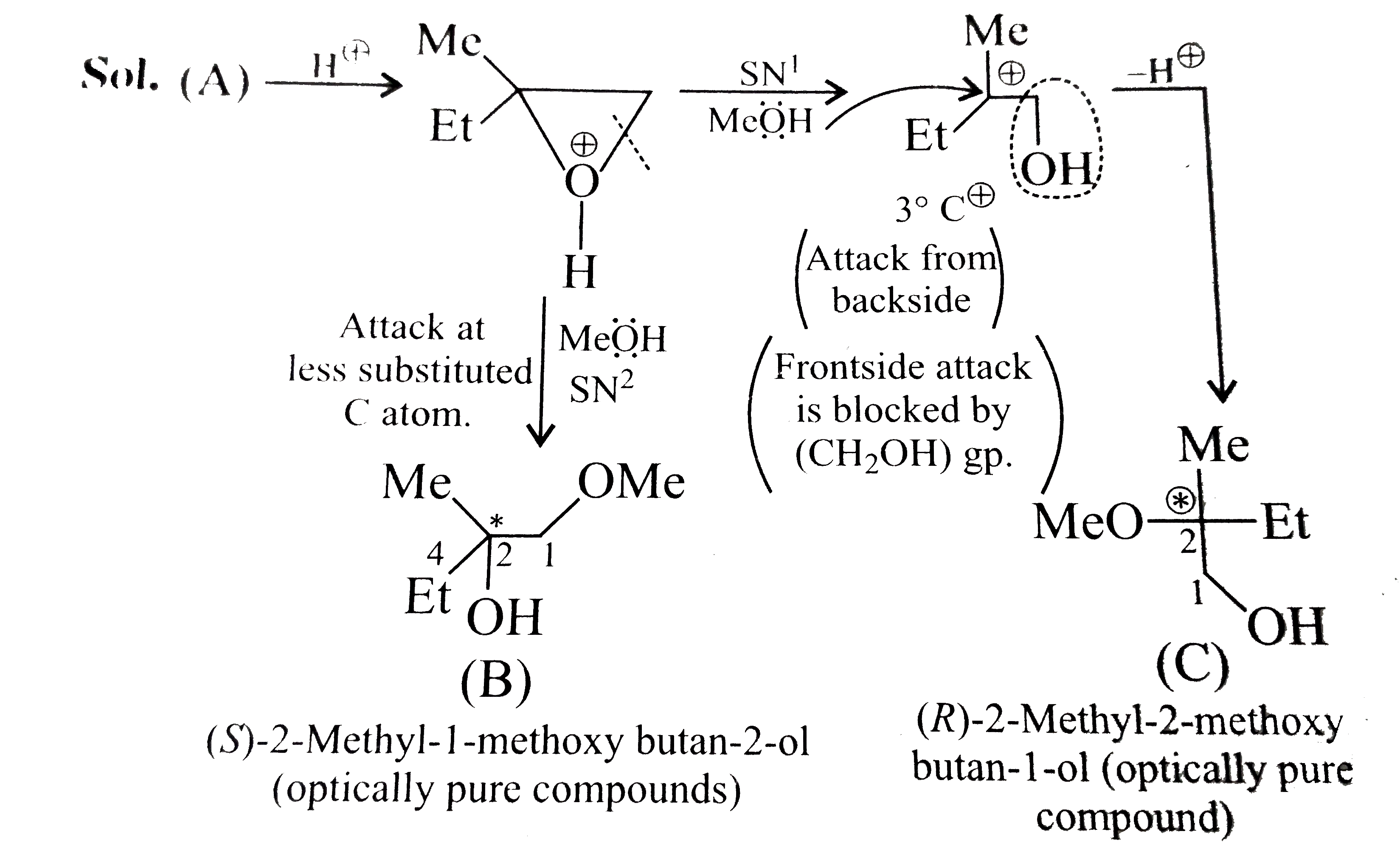
- Explain the formation B and C, optically pure different isomers form (...
Text Solution
|
- An alcohol A, when heated with conc. H(2)SO(4) gives an alkene B. When...
Text Solution
|
- Give reason in one or two sentences form the following: 'o-nitrophenol...
Text Solution
|
- Why is there a large difference in the boiling points of butanal and b...
Text Solution
|
- Complete the following with apporopriate reagent:
Text Solution
|
- Phenol is an acid but does not react with sodium bicarbonate solution...
Text Solution
|
- Indicate steps which would convert: a. Phenol to acetopenone b. ...
Text Solution
|
- An optically active alcohol A (C(6)H(10)O) absorbs 2 mol of hydrogen p...
Text Solution
|
- 2,2-Dimethyloxirane can be cleaved by acid (H^(o+)). Write the mechani...
Text Solution
|
- Consider the following two methods : "Method 1 : "(CH(3))(3)CBr +NaO...
Text Solution
|
- Although phenoxide ion has more number of resonating structures than c...
Text Solution
|
- Acid catalysed dehydration of t-butanol is faster than that of n-butan...
Text Solution
|
- Explain briefly the formation of the product giving structures of the ...
Text Solution
|
- What would be that major product in each of the following reactions ? ...
Text Solution
|
- How would you synthesis 4-methoxyphenol form bromobenzene in NOT more ...
Text Solution
|
- Convert
Text Solution
|