Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Concept Application|33 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|60 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|17 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALCOHOL,PHENOL AND ETHERS-Exercises Subjective
- Compound (A), C(8)H(6)NOBr, is optically active. Reduction of (A) give...
Text Solution
|
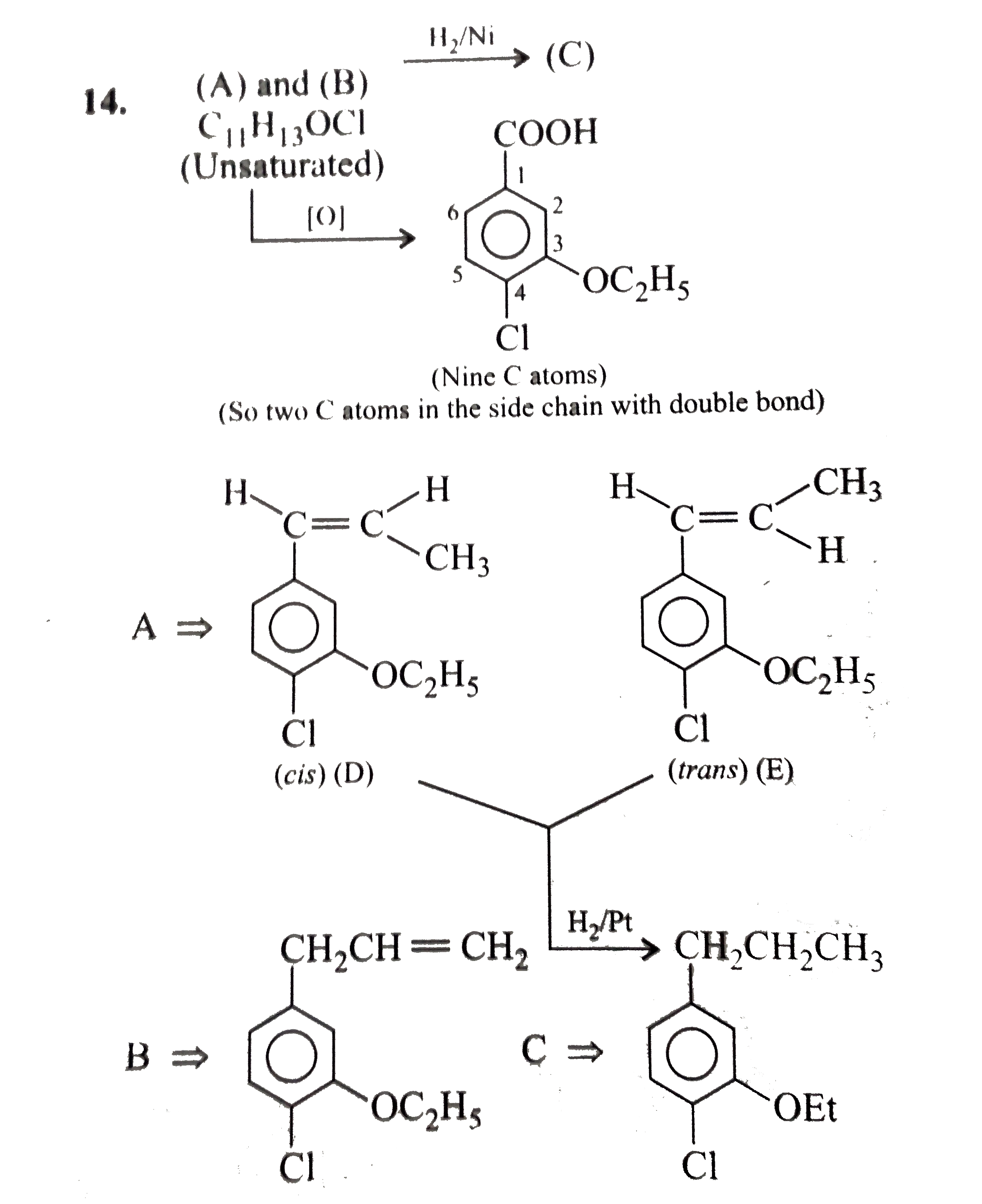
- Identify A to E.
Text Solution
|
- An organic compound (A) (C(8)H(8)O(3)) was insoluble in water, dilute ...
Text Solution
|
- Neutralisation of 30 gm of a mixture of acetic acid and phenol solutio...
Text Solution
|
- Find the structure of (A), C(10)H(10)O(2), a sweet-smelling liquid tha...
Text Solution
|
- a. Isomers (A) and (B), C(10)H(12)O, are isolated form the oil of bay...
Text Solution
|
- An aromatic compound (A), C(7)H(5)NO(2)CI(2) on reduction with Sn//HCI...
Text Solution
|
- An organic compound (A) gives opsitive Liebermann reaction and on trea...
Text Solution
|
- Two isomeric compounds, (A) and (B), have the same formula, C(11)H(13)...
Text Solution
|
- Nirtobenzene is formed as the major product along with a minor product...
Text Solution
|
- Identify A, B, and C, in the following reaction.
Text Solution
|
- Provide a mechanism for the products (A) and (B). Which one will be th...
Text Solution
|
- Identify the substances (A) through (D) in a sequence. Comment on...
Text Solution
|
- Identify each of the following glucose derivative. a. A+5HIO(4)rarr...
Text Solution
|
- If is treated with alkaline NaN(3) and followed by reduction with H(2...
Text Solution
|
- Converst
Text Solution
|
- Assign the structure of (B), the principal organic product of the foll...
Text Solution
|
- When a mixture of t-butyl alcohol and and ethyl alcohol is heated with...
Text Solution
|
- Identify the major products (B) to (H). a.
Text Solution
|
- When pent-4-en-1-ol is treated with aqueous Br(2)//O^(-)H, a cyclic b...
Text Solution
|