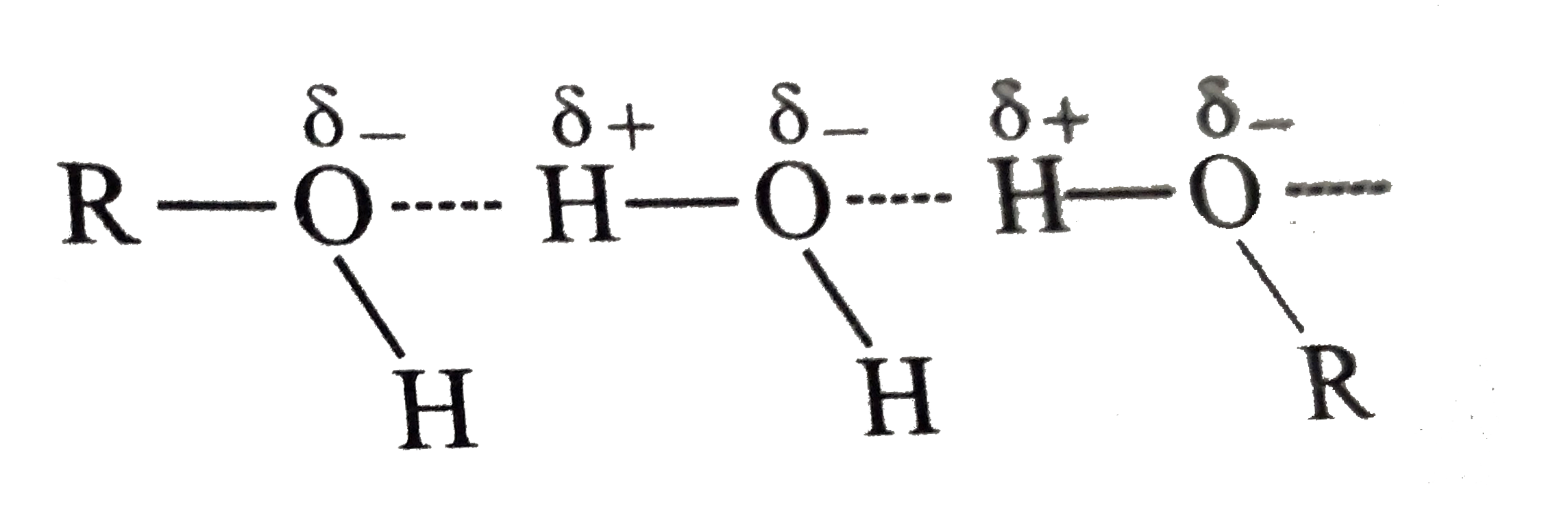Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|60 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|24 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Subjective|25 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALCOHOL,PHENOL AND ETHERS-Exercises Concept Application
- (i) Draw the structures of all isomeric alcohols of molecular formula ...
Text Solution
|
- Explain why propanol has higher boiling point than that of the hydroca...
Text Solution
|
- Alcohols are comparatively more soluble in water than hydrocarbons of ...
Text Solution
|
- What is meant by hydroboration-oxidation reaction? Illustrate it with ...
Text Solution
|
- Give the structures and IUPAC names of monohydric phenols of molecular...
Text Solution
|
- While separating a mixture of ortho- and para-nitrophenols steam disti...
Text Solution
|
- Give the equations of reactions for the preparation of phenol from cum...
Text Solution
|
- Write chemical reaction for the preparation of phenol from chlorobenze...
Text Solution
|
- Write the mechanism of hydration of ethene to yield ethanol.
Text Solution
|
- You are given benzene, conc. H(2)SO(4) and NaOH. Write the equations ...
Text Solution
|
- Show how will you synthesise: (i) 1-phenylethanol from a suitable al...
Text Solution
|
- Give two reactions that show the acidic nature of phenol. Compare acid...
Text Solution
|
- Explain why is ortho nitrophenol more acidic than ortho methoxyphenol ...
Text Solution
|
- Explain how does the –OH group attached to a carbon of benzene ring ac...
Text Solution
|
- Give equations of the following reactions: (i) Oxidation of propan-1...
Text Solution
|
- Explain the following with an example: i. Kolbe's reaction ii. R...
Text Solution
|
- Write the mechanism of hydration of ethene to yield ethanol.
Text Solution
|
- How are the following conversions carried out? (i) Propene rarr Pro...
Text Solution
|
- Name the reagents used in the following reactions: (i) Oxidation of ...
Text Solution
|
- Give reason for the higher boiling point of ethanol in comparison to m...
Text Solution
|