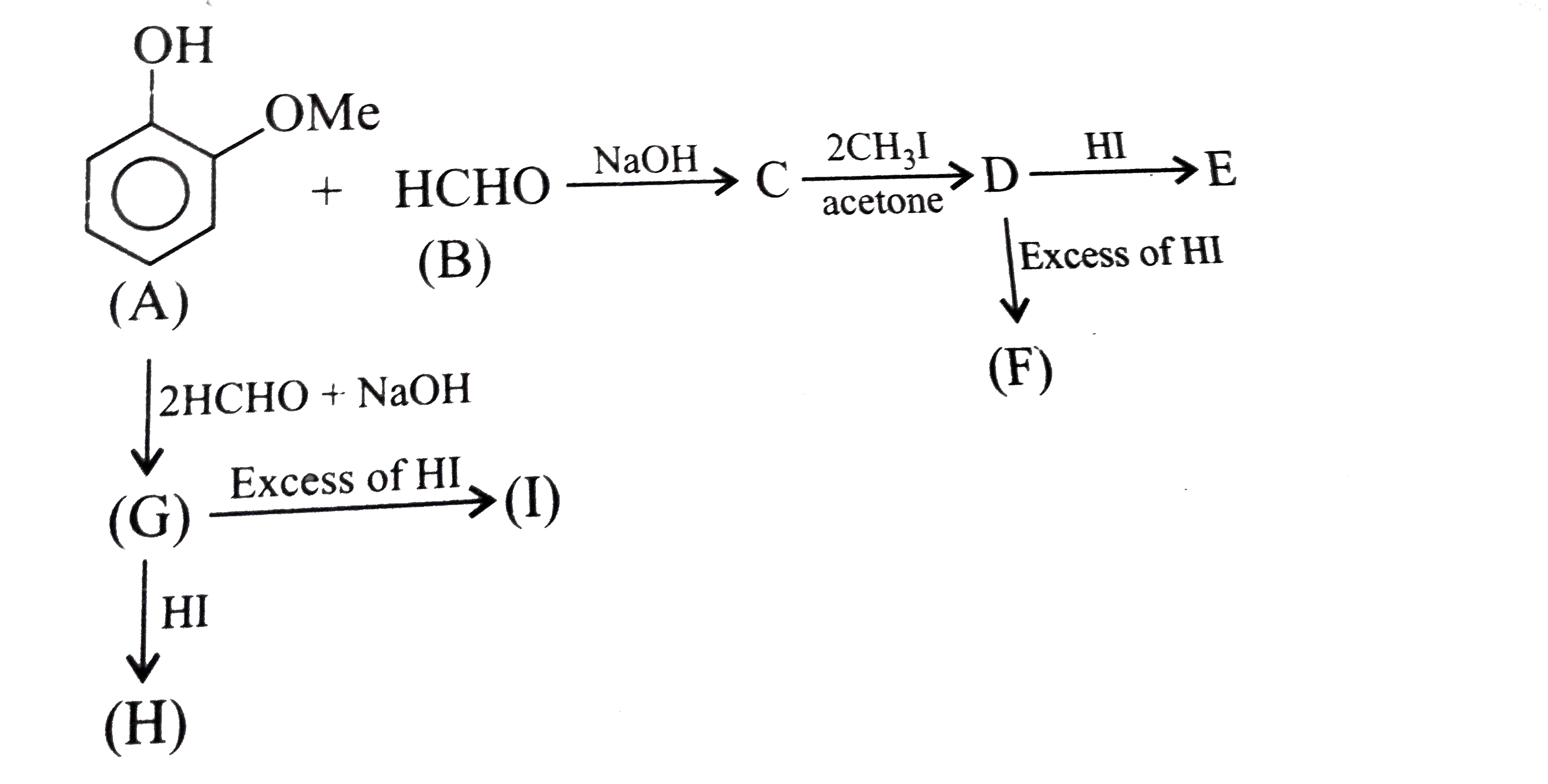
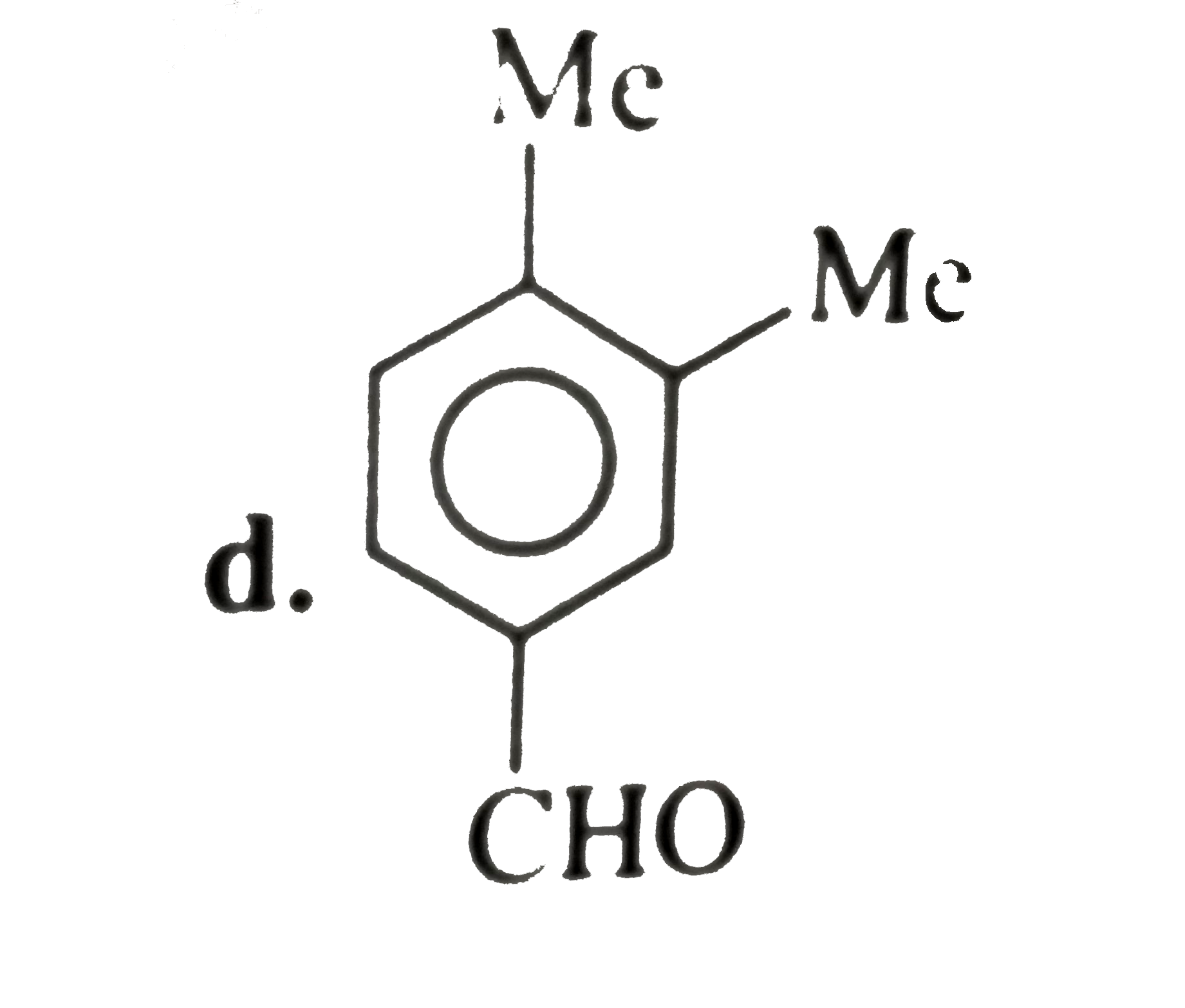
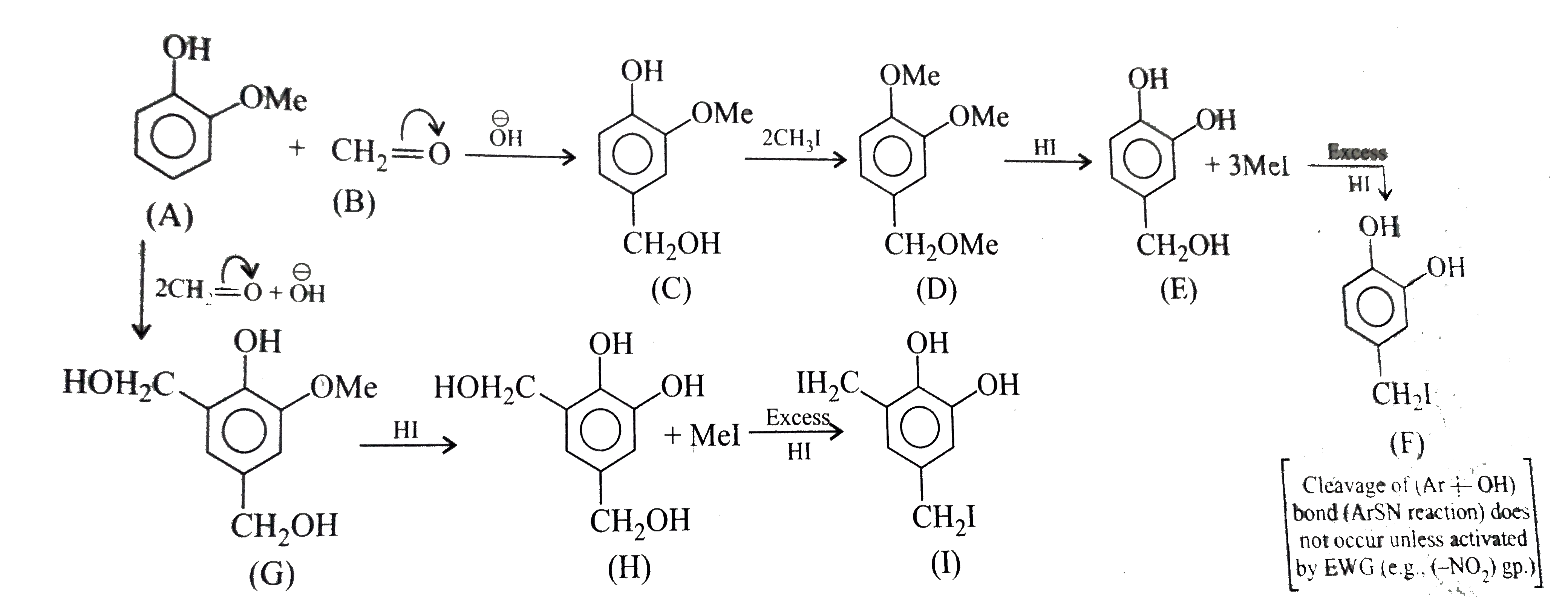
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|24 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|72 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Concept Application|33 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALCOHOL,PHENOL AND ETHERS-Exercises Linked Comprehension
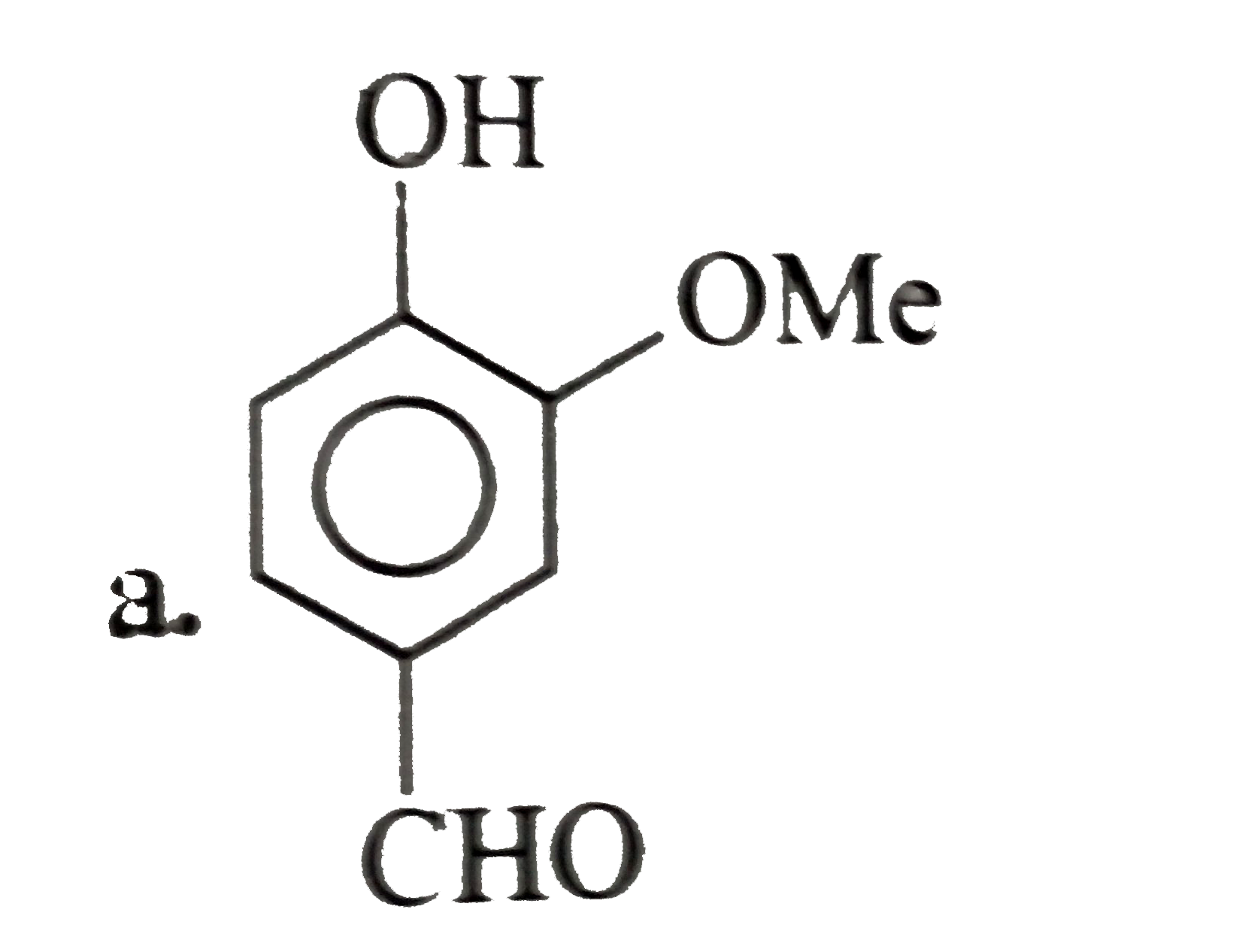
- The compound (C ) is:
Text Solution
|
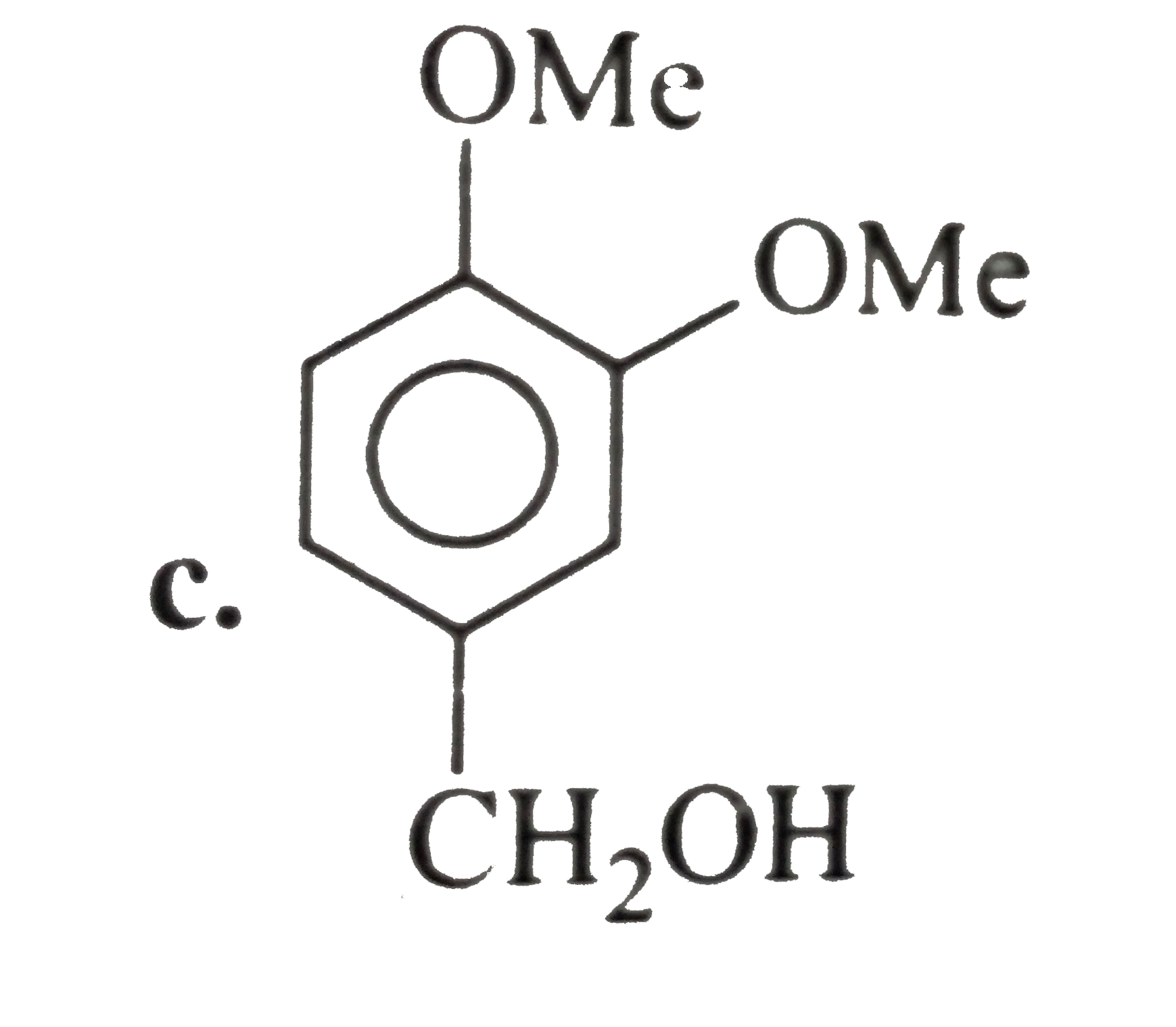
- The compound (D) is:
Text Solution
|
- The compound (E ) is:
Text Solution
|
- The compound (F) is:
Text Solution
|
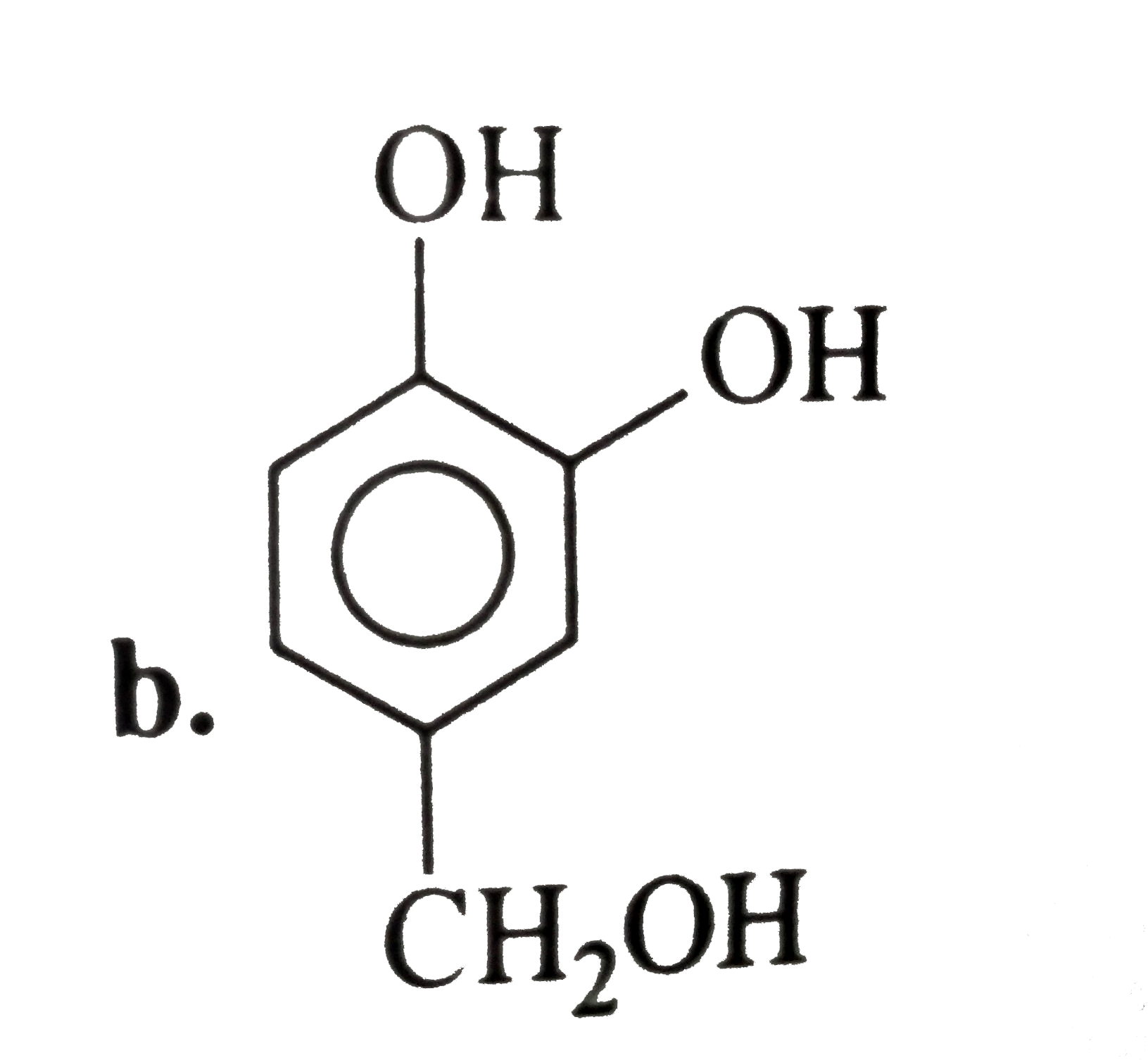
- The compound (G) is:
Text Solution
|
- The compound (H) is:
Text Solution
|
- The compound (C) is:
Text Solution
|
- What is the product B?
Text Solution
|
- The major product of (B) is?
Text Solution
|
- Which of the following does not give iodoform/haloform reaction ?
Text Solution
|
- The compound (C ) is:
Text Solution
|
- The compound (D) is:
Text Solution
|
- The compound (B) is:
Text Solution
|
- The compound (C ) is:
Text Solution
|
- The compound (D) is:
Text Solution
|
- The compound (G) and (H), respectively, are:
Text Solution
|
- The compound (E ) is:
Text Solution
|
- The compound (F) is:
Text Solution
|
- The common name of the compound (D) is:
Text Solution
|
- The schotten-Baumann reaction is:
Text Solution
|