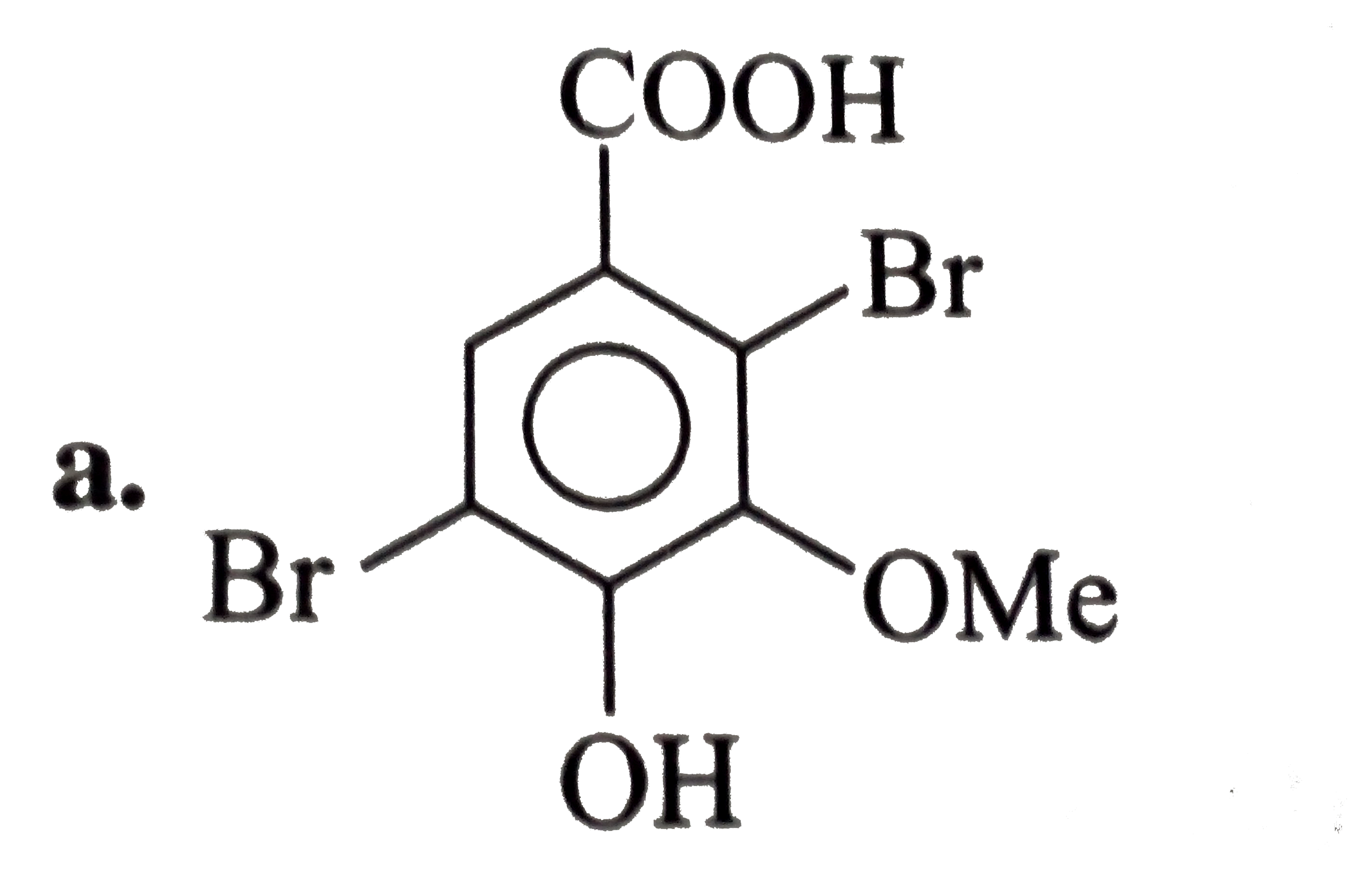
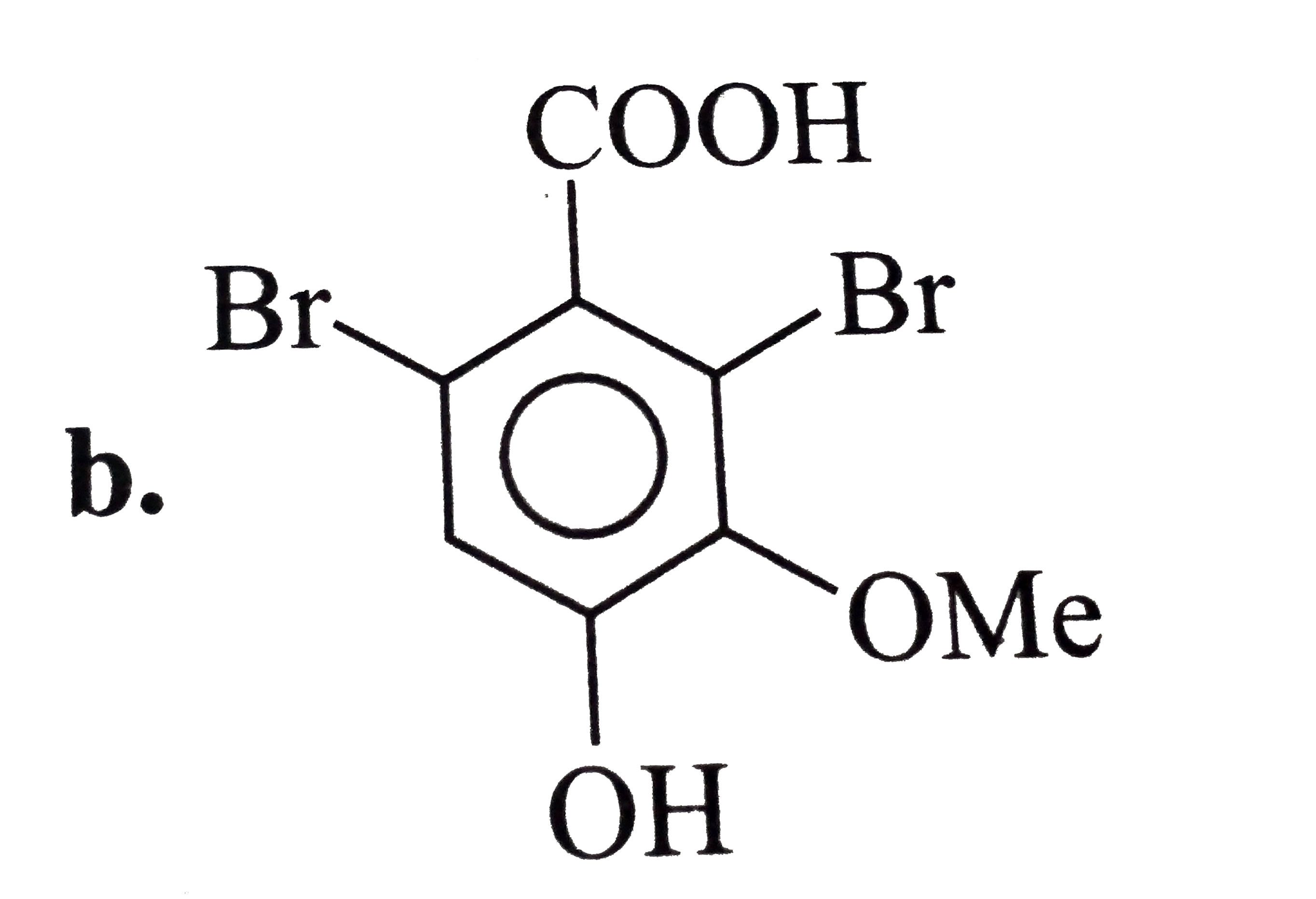
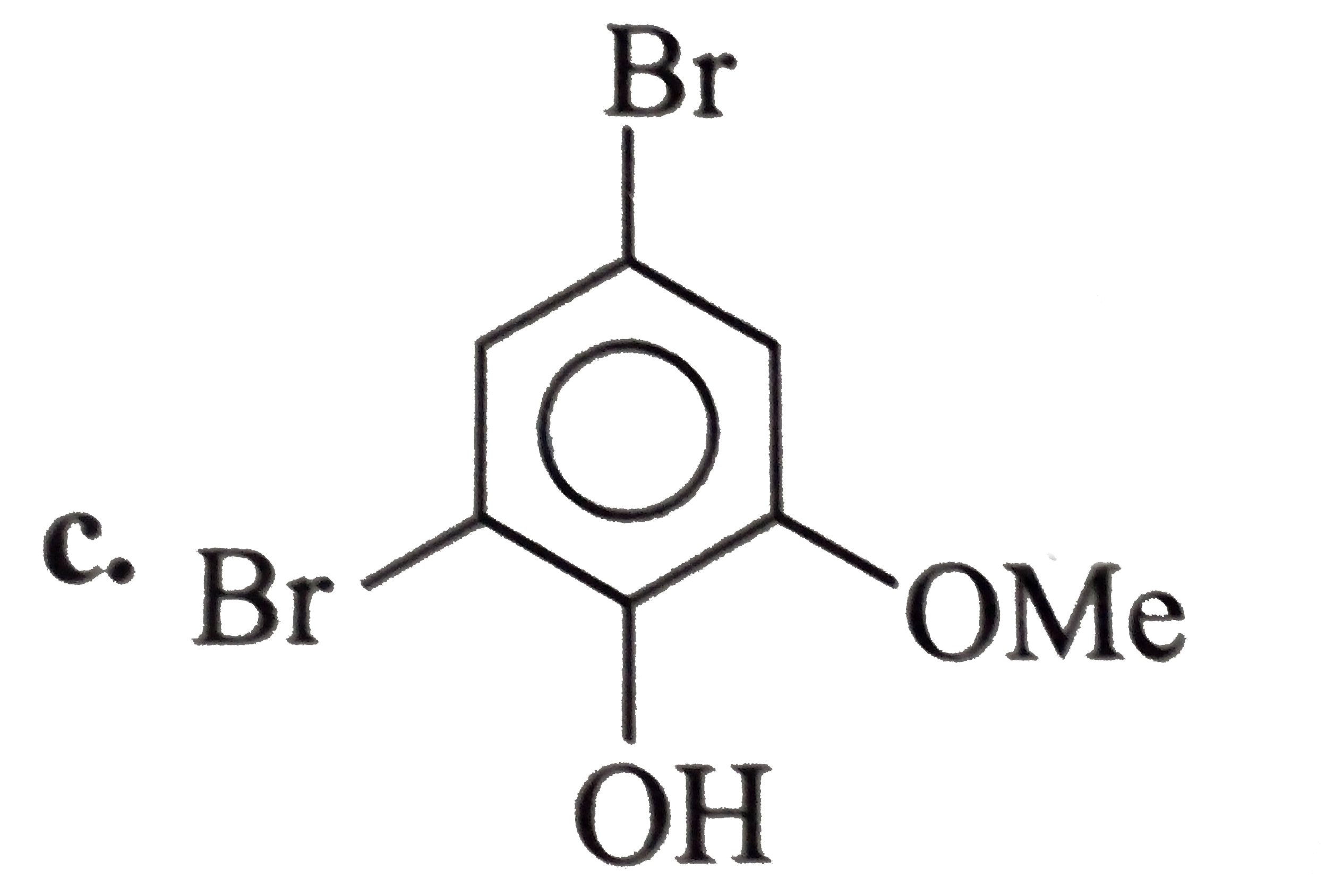
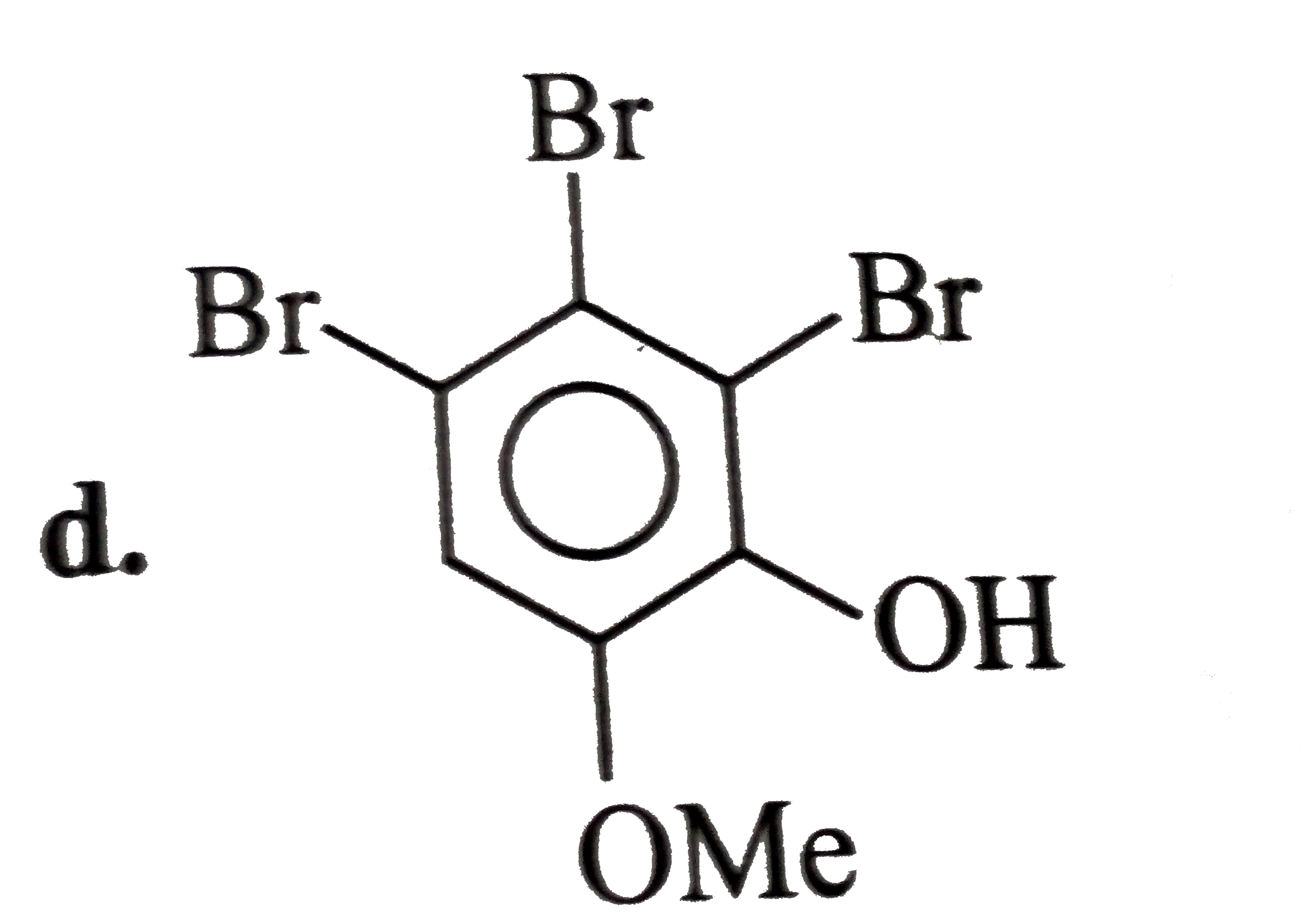
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|24 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|72 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Concept Application|33 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALCOHOL,PHENOL AND ETHERS-Exercises Linked Comprehension
- The expected structure of A is?
Text Solution
|
- (a)Give a chemical test to distinguish between saturated and unsaturat...
Text Solution
|
- What physical and chemical properties of elements were used by Mendele...
Text Solution
|
- The compound (C ) is:
Text Solution
|
- The names of reaction and intermediate species involved in the formati...
Text Solution
|
- The compound (D) is:
Text Solution
|
- The compound (F) and (G) are geometrical isomers. The stable form is:
Text Solution
|
- The compound (H) is:
Text Solution
|
- The reaction and mechanism involved in the formation of compound (H) f...
Text Solution
|
- The compound (I) is
Text Solution
|
- The reaction and mechanism involved in the formation of the compound (...
Text Solution
|
- The compound (C ) is:
Text Solution
|
- Convert Propanoic acid into Ethanoic acid.
Text Solution
|
- The compound C is
Text Solution
|
- State True or False Copper reacts with dilute Hydrochloric acid to l...
Text Solution
|
- The compound (H) is:
Text Solution
|
- The reaction (C ) to (D) and (E ) is called alkoxy mercuration-demercu...
Text Solution
|
- The reaction (C ) to (F and G) is called mercuration-demercuration rea...
Text Solution
|
- The reaction (C ) to (H) is called hydroboration oxidation of alkene. ...
Text Solution
|
- Which of the following statements is wrong about hydroboration oxidati...
Text Solution
|