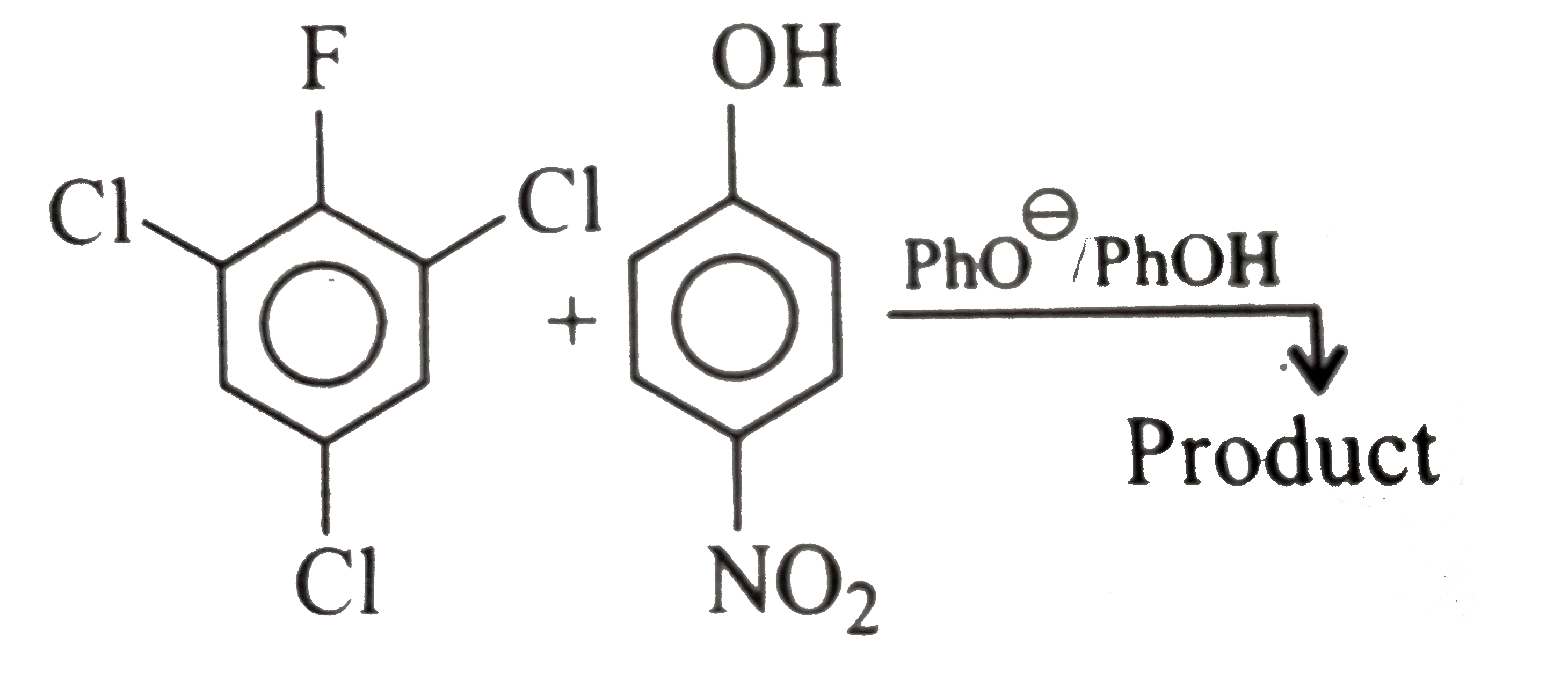
A
B
C
D
Text Solution
Verified by Experts
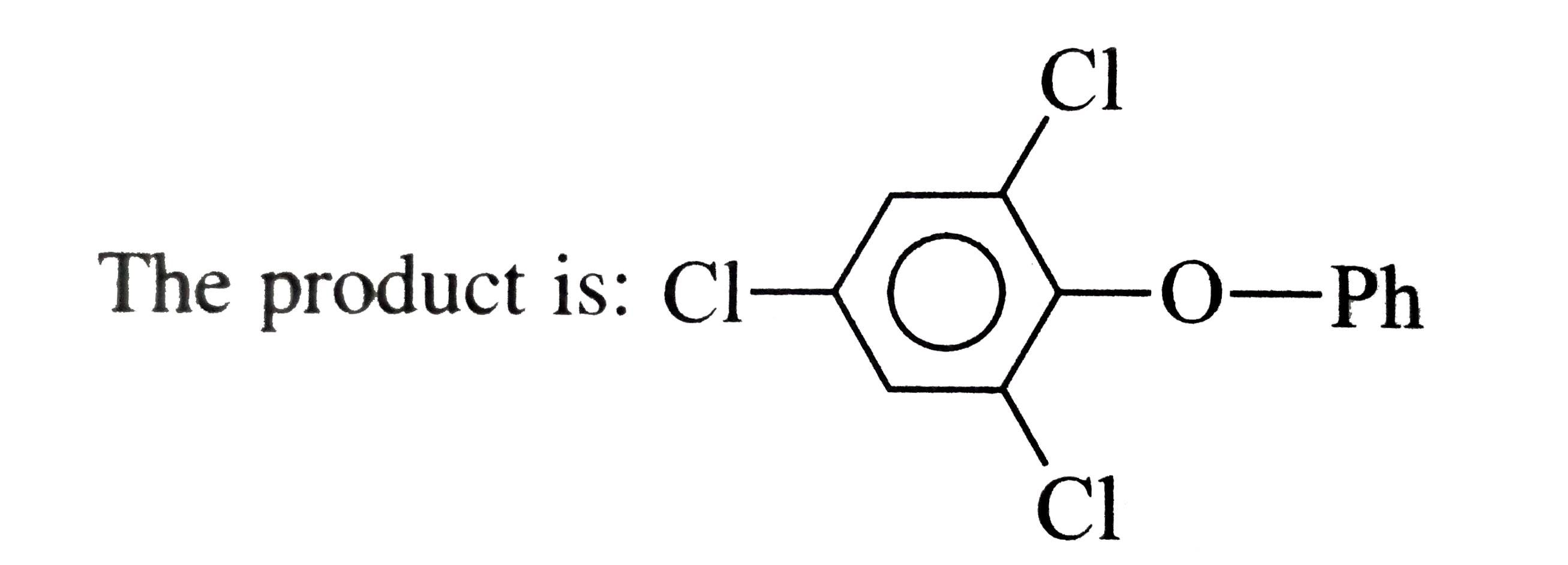
The correct Answer is:
Topper's Solved these Questions
ALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Single Correct|14 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Assertion-Reasoning|2 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|72 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALCOHOL,PHENOL AND ETHERS-Exercises Assertion-Reasoning
- Assertion (A): The major products formed by heating with HI are: ...
Text Solution
|
- Assertion (A): The major products formed by heating compound (A) in Q....
Text Solution
|
- Assertion (A): The boiling point of diethyl ether is greater than fura...
Text Solution
|
- Assertion (A): The product is: Reason (R ): p-NO(2)-C(6)H(4)O^(-...
Text Solution
|
- Assertion (A): 2,6-Dimethyl-4-nitrophenol (I) is more acidic than 3,5-...
Text Solution
|