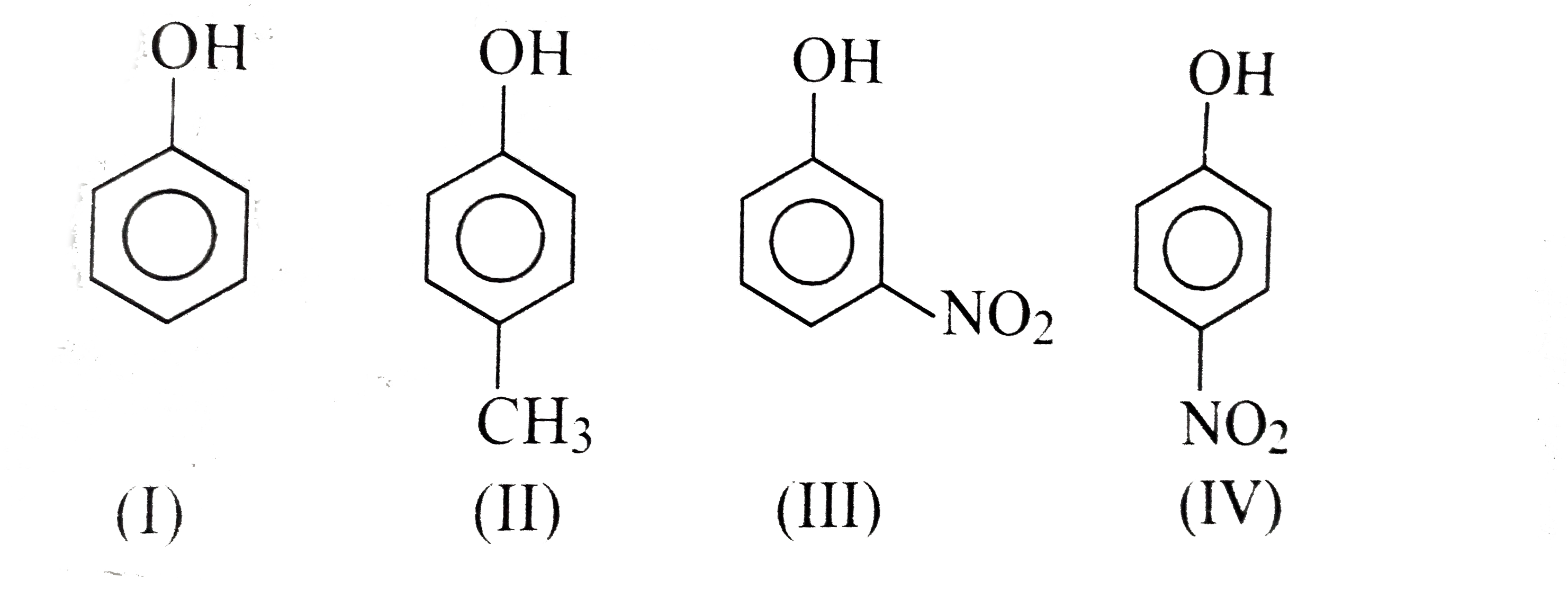
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Assertion-Reasoning|2 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Linked Comprehension|2 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Reasoning|5 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALCOHOL,PHENOL AND ETHERS-Archives Single Correct
- Ethyl alcohol is heated with conc. H(2)SO(4) at 170^(@)C. The product ...
Text Solution
|
- The compound that reacts fastest with Lucas reagent at room temperatu...
Text Solution
|
- Diethyl ether on heating with conc. HI gives two moles of:
Text Solution
|
- An industrial method for preparation of methanol is:
Text Solution
|
- HBr reacts fastest with
Text Solution
|
- Hydrogen bonding is maximum in:
Text Solution
|
- In CH(3)CH(2)OH, the bond that undegoes heterolytic clevage most read...
Text Solution
|
- The products of combustion of an aliphatic thiol (RSH) at 298K are
Text Solution
|
- In the following compounds, the order of acidity is:
Text Solution
|
- Benzenediazonium chloride on reaction with phenol in weakly basic medi...
Text Solution
|
- When phenyl magnesium bromide reacts with t-butanol the product would ...
Text Solution
|
- The best method to prepare cyclohexene form cyclohexanol is by using:
Text Solution
|
- Consider the following compounds: (I) 1, 2-dihydroxy benzene (II)...
Text Solution
|
- In the reaction OCH(3)overset(HBr)rarr the product are:
Text Solution
|