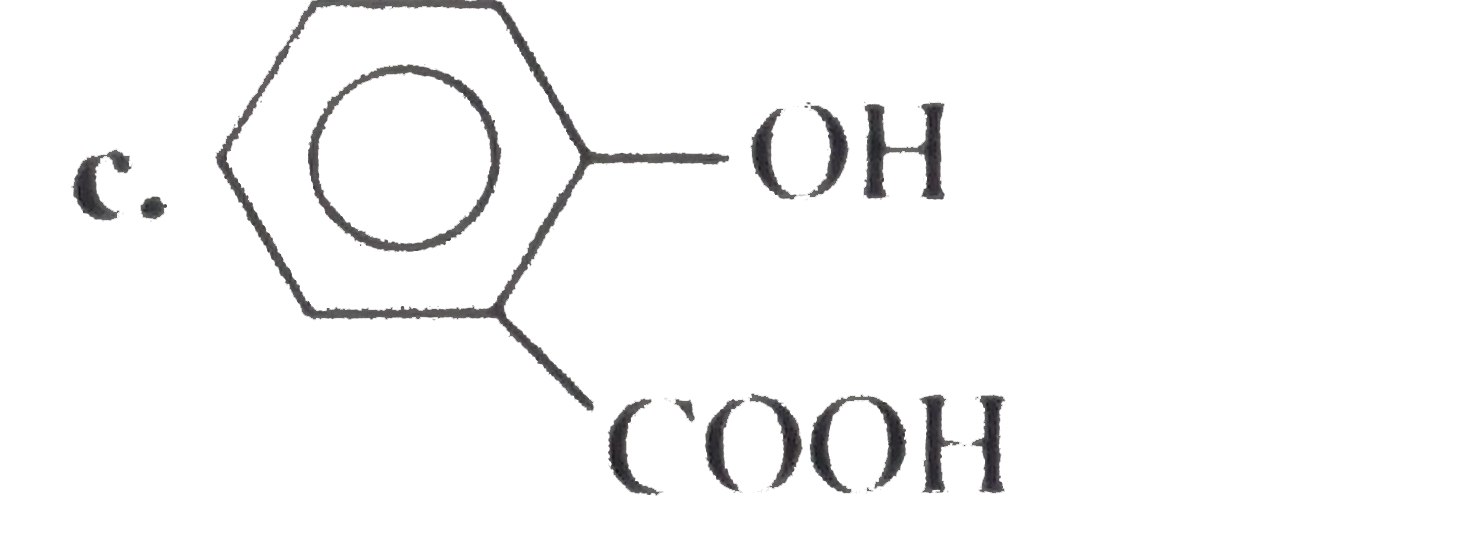
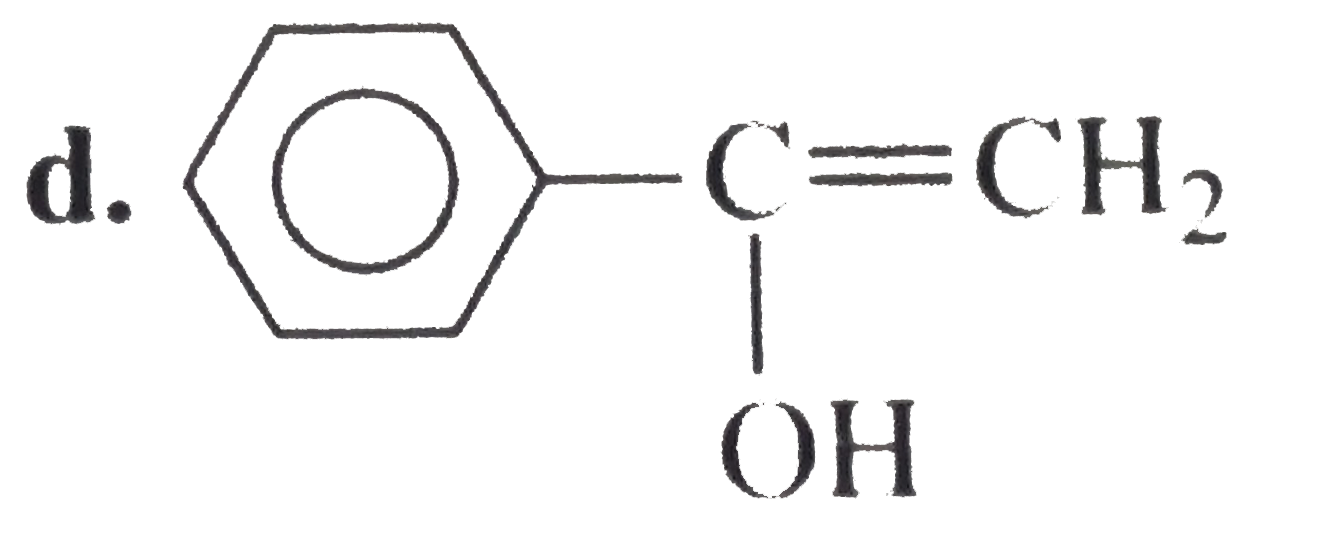
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion-Reasoning)|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Single Correct)|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct)|34 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|8 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Exercises (Singlecorrect)
- The decreasing order of ester formation with benzoic acid in presence ...
Text Solution
|
- The decreasing order of ester formation with acetic acid in presence o...
Text Solution
|
- Which of the following cannot be acetylated with CH3 COCl//Py ?
Text Solution
|
- Which of the following is the strongest acid ?
Text Solution
|
- What is the effect of reducing the volume on the system described belo...
Text Solution
|
- Which reagent can distinguish between pentanoic acid and pentanamide ?
Text Solution
|
- The compound (D) is :
Text Solution
|
- the standard heat of formation of Fe2O3 (s) is 824.2kJ mol-1 Calculate...
Text Solution
|
- Which H atom in the following ester is most acidic ? overset(1)(CH(3...
Text Solution
|
- Product (B) in the reaction is : .
Text Solution
|
- The reactant (A) is the reaction is : .
Text Solution
|
- Product ( C) is :
Text Solution
|
- Product ( C) is :
Text Solution
|
- Give two examples of reactions which are driven by enthalpy change.
Text Solution
|
- The decreasing order of ease of hydrolysis of the following esters is ...
Text Solution
|
- The boiling points of ethyl acetate and CH3 OH, respectively, are 77^@...
Text Solution
|
- Carboxylic acid, although unreactive to alcohols, reacts in the presen...
Text Solution
|
- The major product ( C) in the reaction is : .
Text Solution
|
- Which of the following is the best method for the synthesis of ester (...
Text Solution
|
- Which of the following is the best method for the synthesis of alpha-h...
Text Solution
|