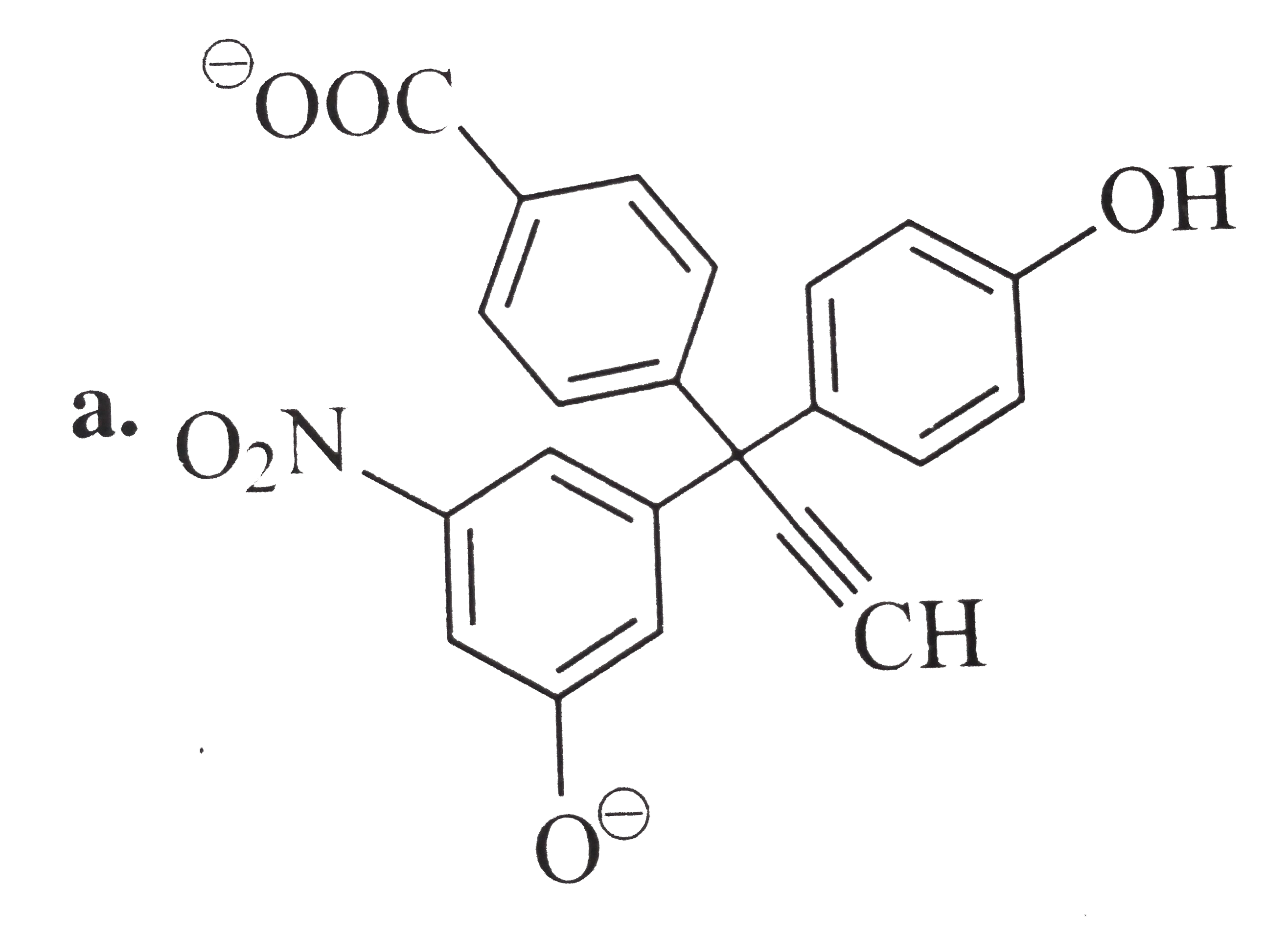
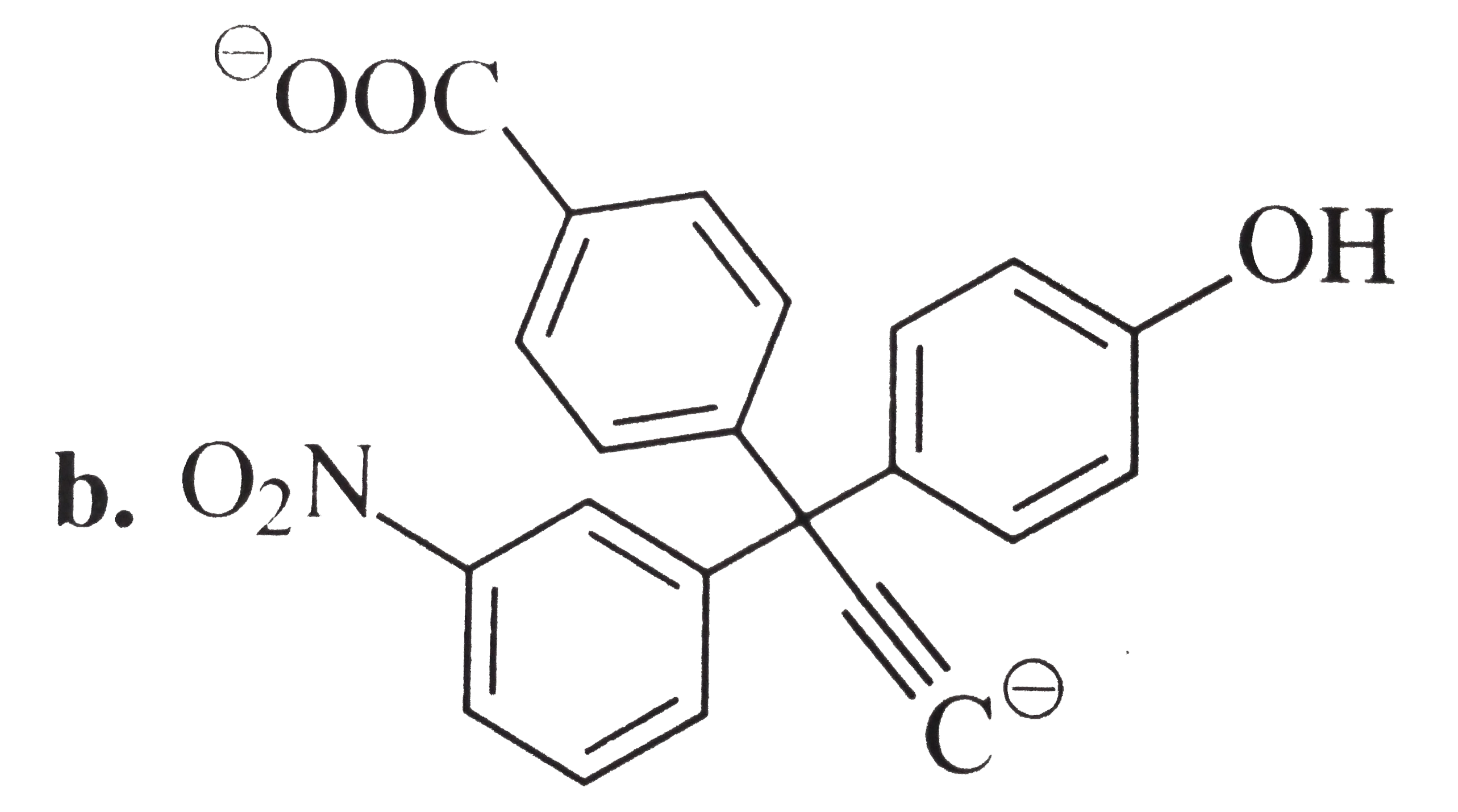
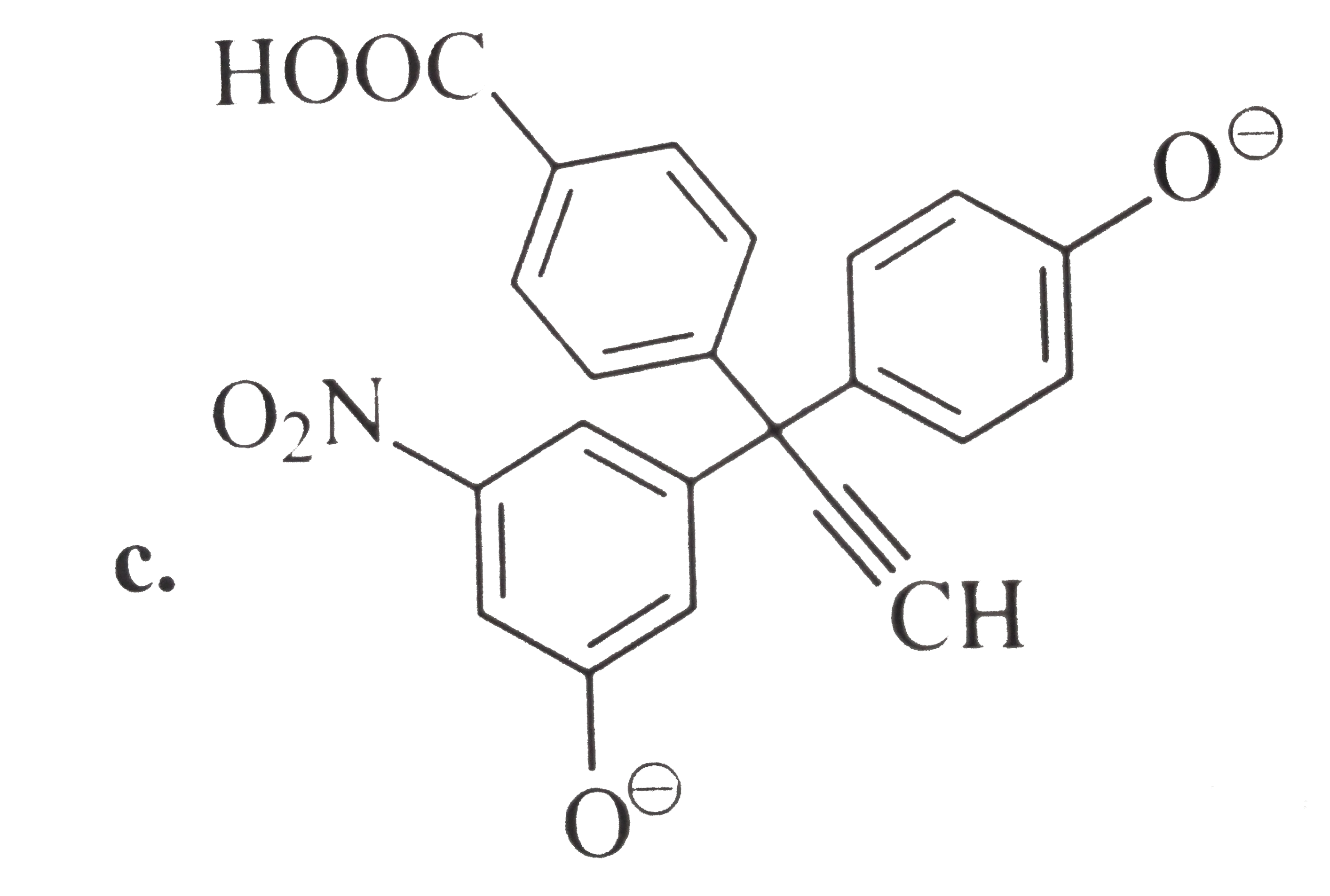
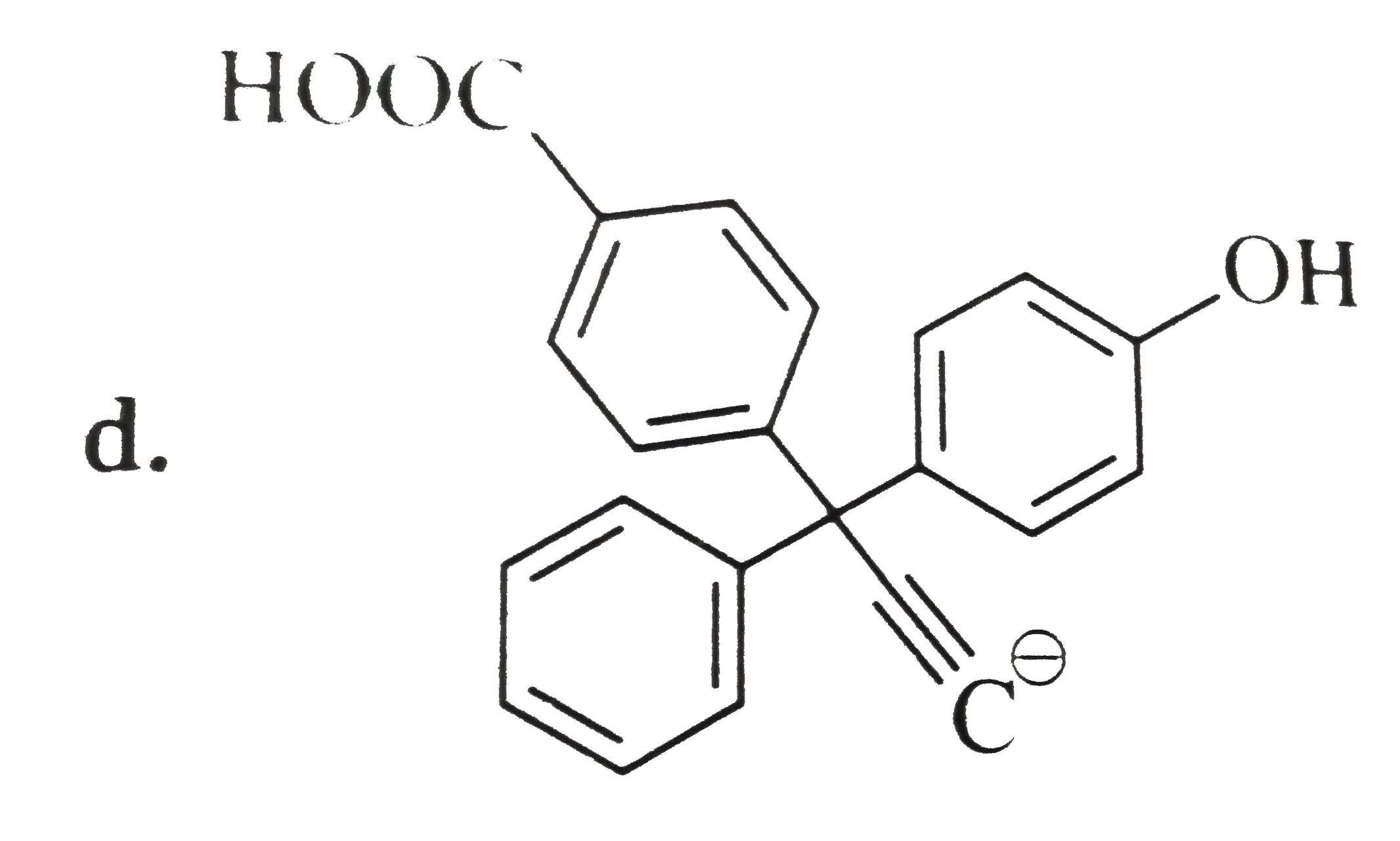
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Assertion-Reasoning)|2 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Linked Comprehension)|3 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion-Reasoning)|10 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|8 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 Videos
CENGAGE CHEMISTRY ENGLISH-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Exercises Archives (Single Correct)
- Which of the following is basic ?
Text Solution
|
- Acetamide is treated with the following reagents separately. Which one...
Text Solution
|
- Hydrogenation of benzoyl chloride in the presence of Pd on BaSO(4) giv...
Text Solution
|
- When propionic acid is treated with aqueous sodium bicarbonate, CO(2) ...
Text Solution
|
- Benzoyl chloride is prepared from benzoic acid by
Text Solution
|
- Identify the correct order of boiling points of the following compound...
Text Solution
|
- .
Text Solution
|
- An enantiomerically pure is treated with racemic mixture of an alcohol...
Text Solution
|
- Benzamide on treatment with POCl(3) gives
Text Solution
|
- Which benzene sulphonic acid and p-nitrophenol are treated with NaHCO3...
Text Solution
|
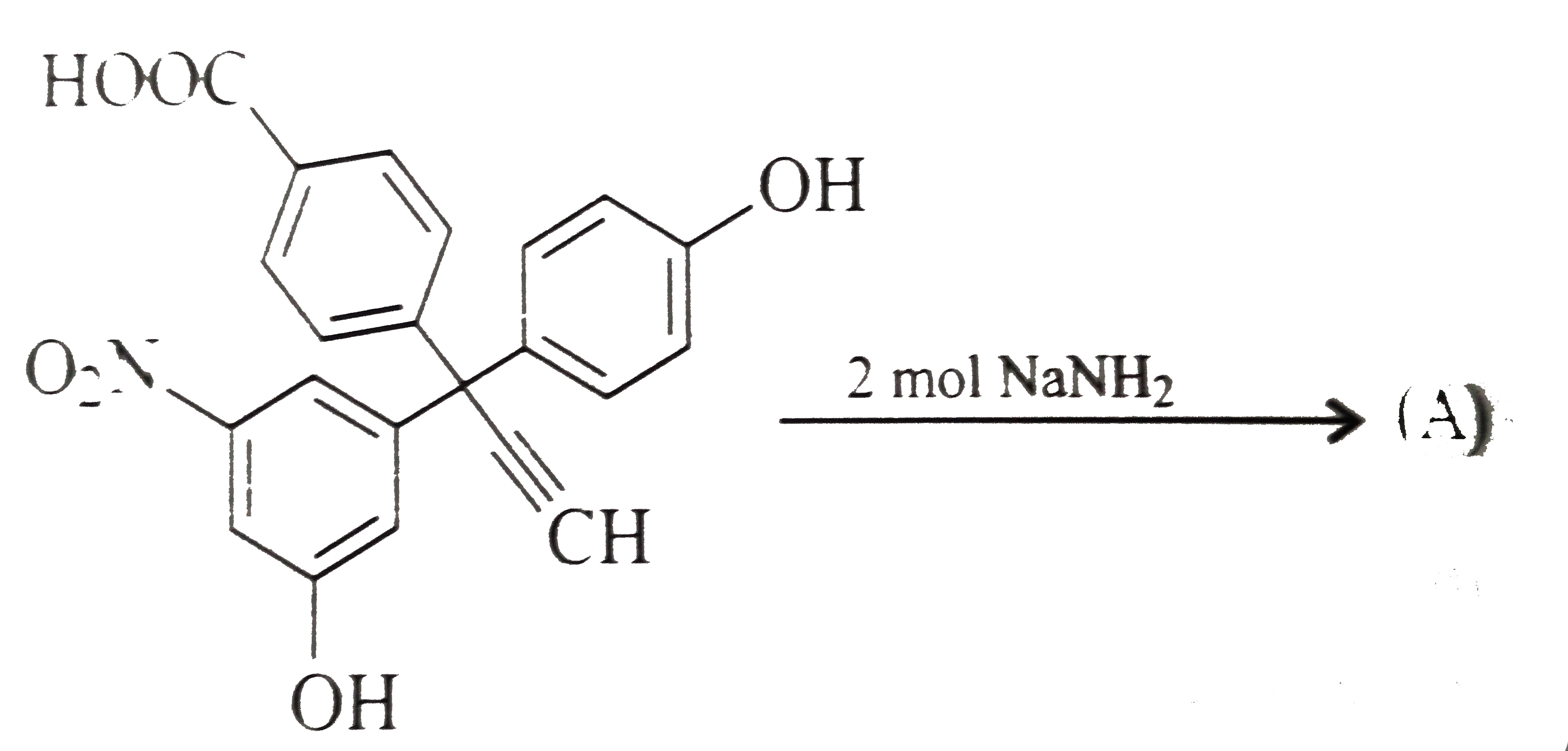 .
.