Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|18 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|51 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise concept application 4|1 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|13 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP-Exercises Linked Comprehension
- What is water – gas shift reaction?
Text Solution
|
- Hard water does not produce lather with soap. True/False
Text Solution
|
- T strength in volumes of a solution containing 30.36 g/l of H2O2 will ...
Text Solution
|
- Explain why cation are smaller and anions larger in radii than their p...
Text Solution
|
- Is it possible to acidify borax solution?
Text Solution
|
- Write balanced equations to show hydrolysis reactions of CO3^2^- and H...
Text Solution
|
- Boric acid is a strong acid. True/False
Text Solution
|
- Write reaction when B(OH)3 is heated.
Text Solution
|
- [A],[B],[C],[D],[E],[F],and [G] are amines,each of which forms a hydro...
Text Solution
|
- [A],[B],[C],[D],[E],[F],and [G] are amines,each of which forms a hydro...
Text Solution
|
- [A],[B],[C],[D],[E],[F],and [G] are amines,each of which forms a hydro...
Text Solution
|
- [A],[b],[C],[D],[E],[F],and[G] are amines,each of which forms a hydroc...
Text Solution
|
- [A],[b],[C],[D],[E],[F],and[G] are amines,each of which forms a hydroc...
Text Solution
|
- [A],[b],[C],[D],[E],[F],and[G] are amines,each of which forms a hydroc...
Text Solution
|
- A substance(X) contains 41.37% C,6.89% H.0.166gm of (X) gaveNH(3) whic...
Text Solution
|
- A substance(X) contains 41.37% C,6.89% H.0.166gm of (X) gaveNH(3) whic...
Text Solution
|
- A substance(X) contains 41.37% C,6.89% H.0.166gm of (X) gaveNH(3) whic...
Text Solution
|
- A substance(X) contains 41.37% C,6.89% H.0.166gm of (X) gaveNH(3) whic...
Text Solution
|
- A substance(X) contains 41.37% C,6.89% H.0.166gm of (X) gaveNH(3) whic...
Text Solution
|
- A substance(X) contains 41.37% C,6.89% H.0.166gm of (X) gaveNH(3) whic...
Text Solution
|
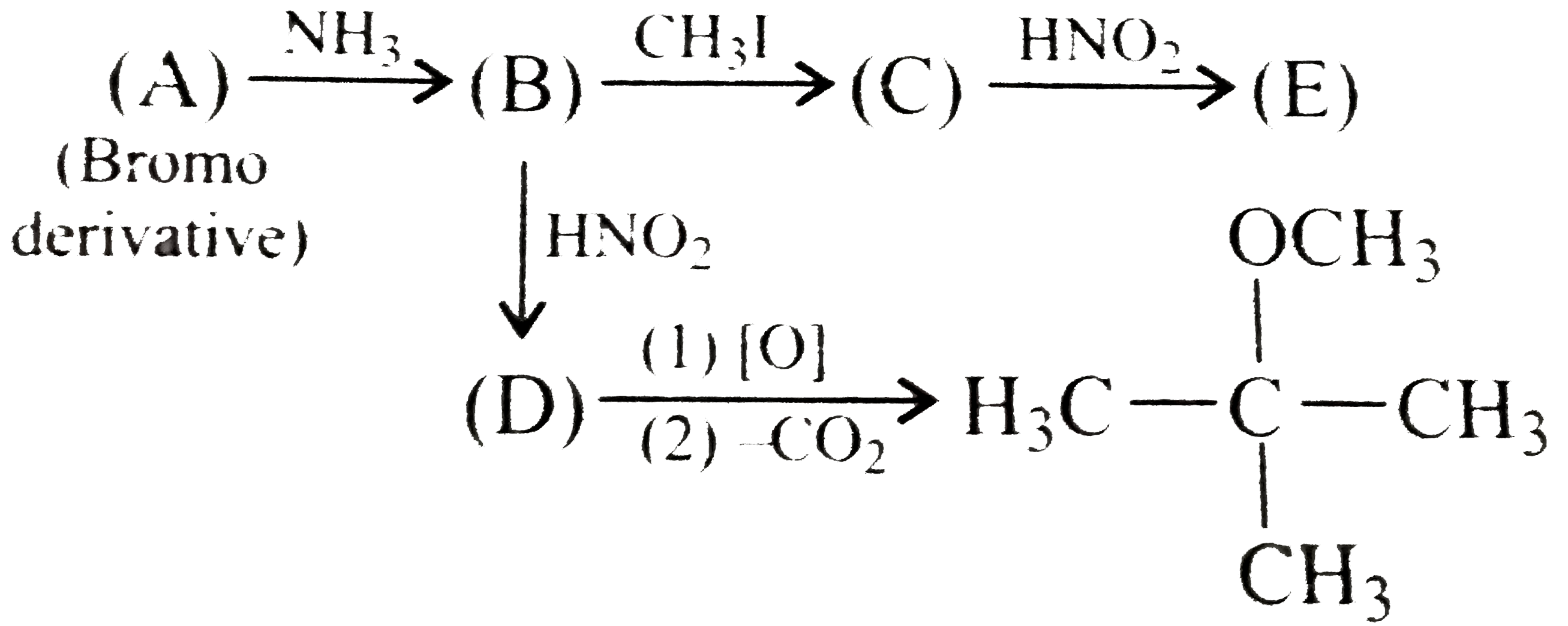
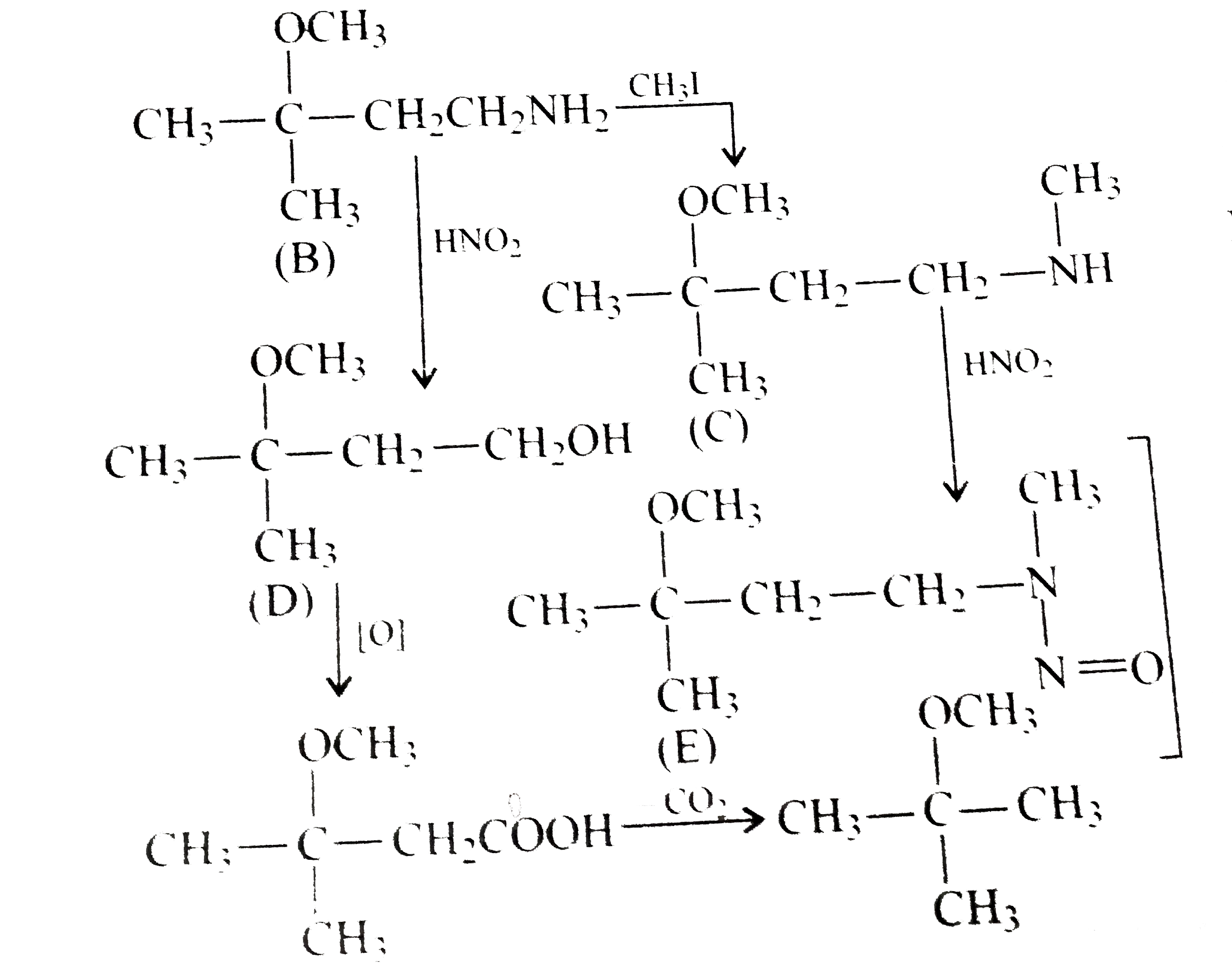 .
.