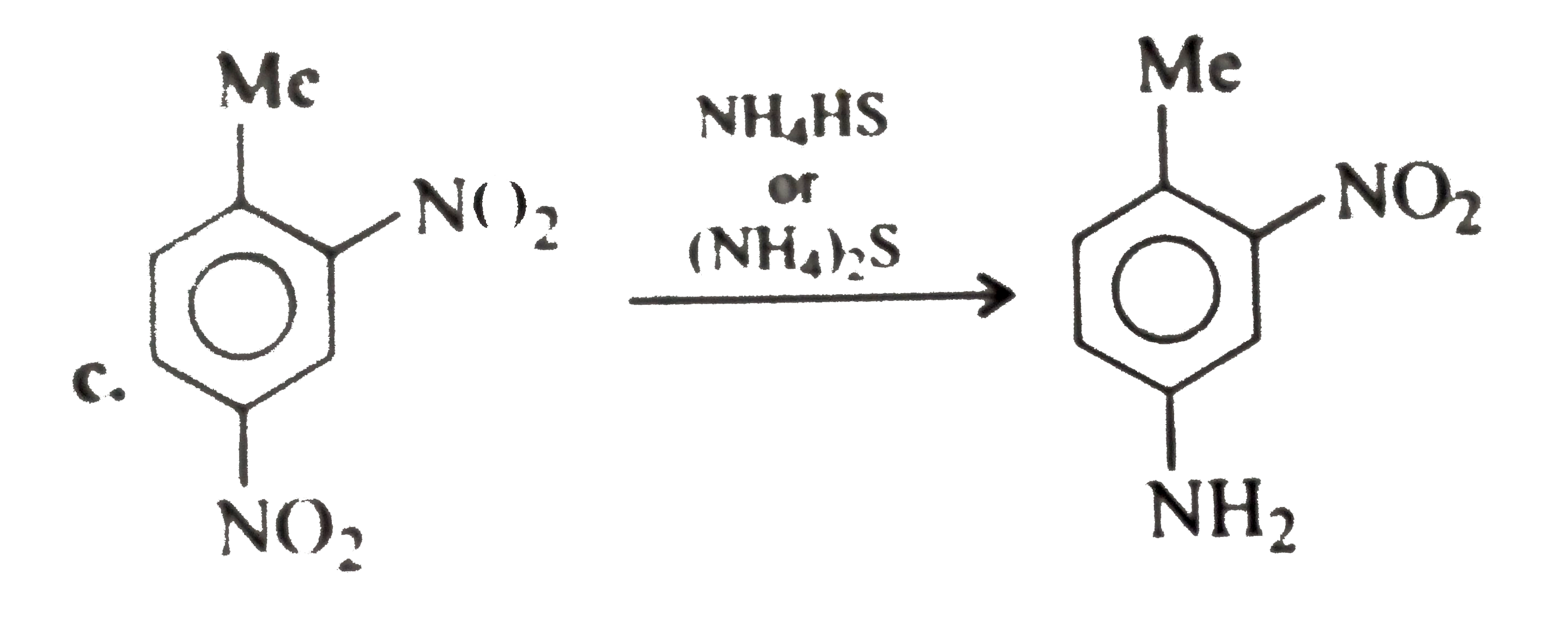
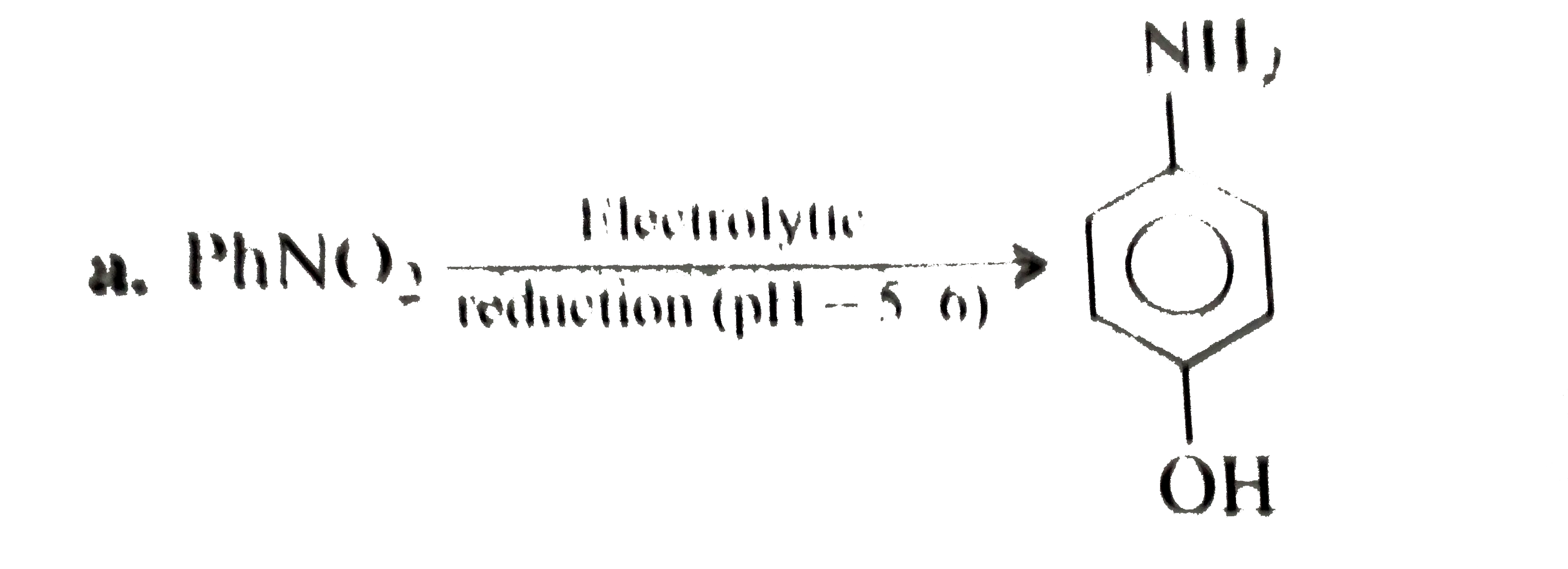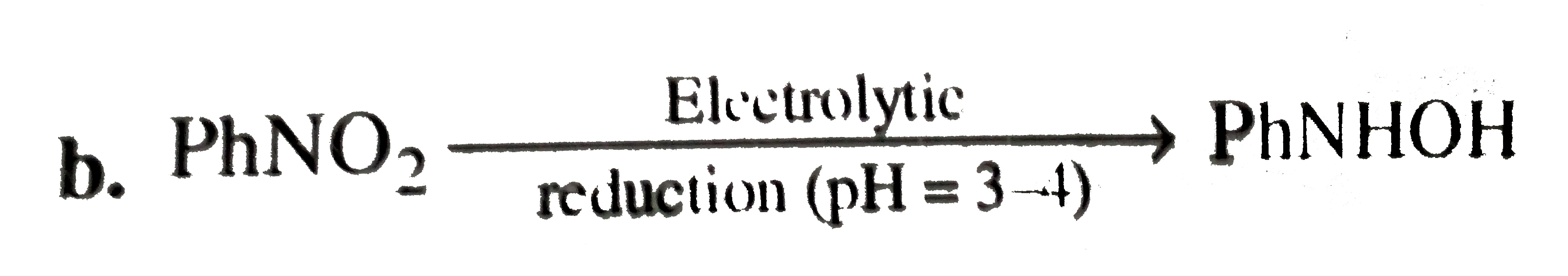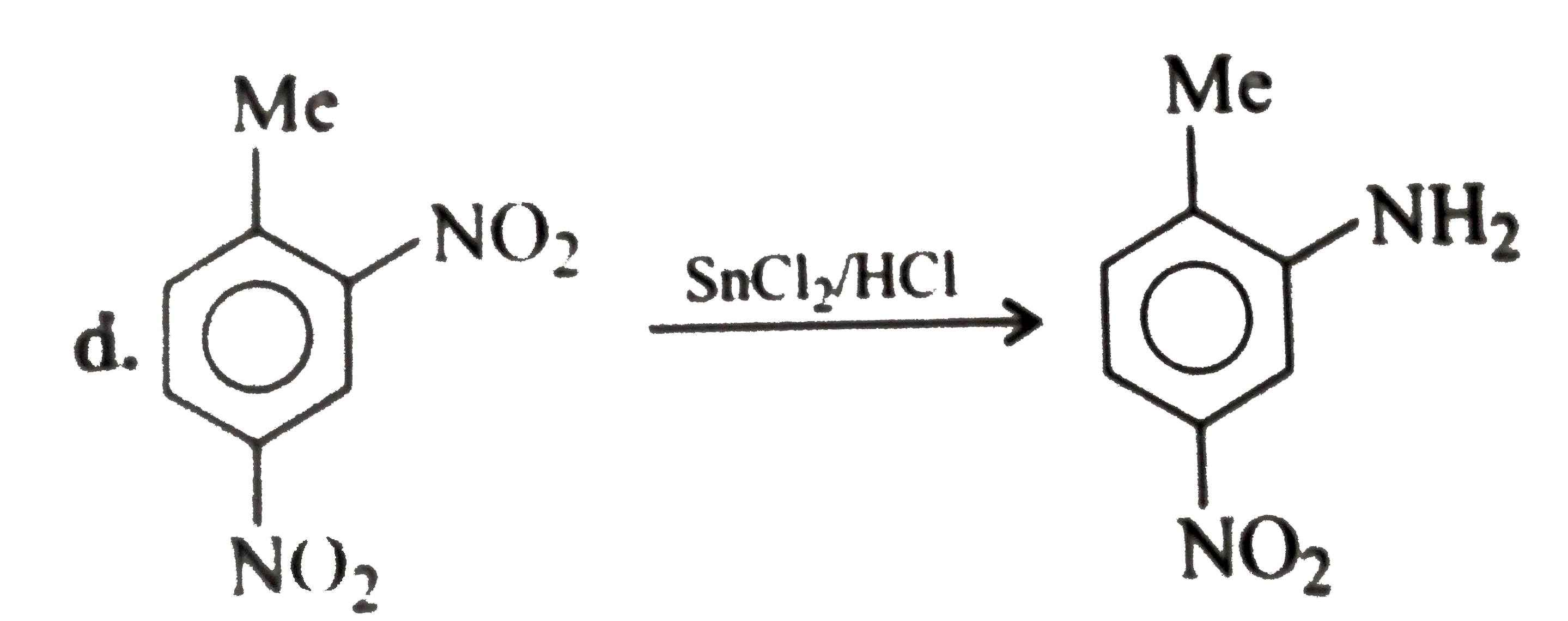A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|51 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion And Reasoning|9 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|25 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|13 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP-Exercises Multiple Correct
- Which statements are correct ?
Text Solution
|
- Carbon forms compounds whereas lead forms compounds.
Text Solution
|
- Which of the following statement are correct reactions?
Text Solution
|
- What of the following statements are correect?
Text Solution
|
- Which of the following reaction are correct?
Text Solution
|
- Which of the following statement are correct?
Text Solution
|
- Which of the following are the correct orders of basic character?
Text Solution
|
- Which statements are correct about MIC (methyl isocyanate, Me-N=C=O).
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following reagents are correct for the given reaction?
Text Solution
|
- Which of the following would give Hofmann alkene?
Text Solution
|
- Which of the following are Cope reactions?
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- By which of the following reactions can MIC (methyl isocyante) be obta...
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- The smell of perfume spreads over the room due to the property called....
Text Solution
|
- Which of the following reaction are wrong?
Text Solution
|