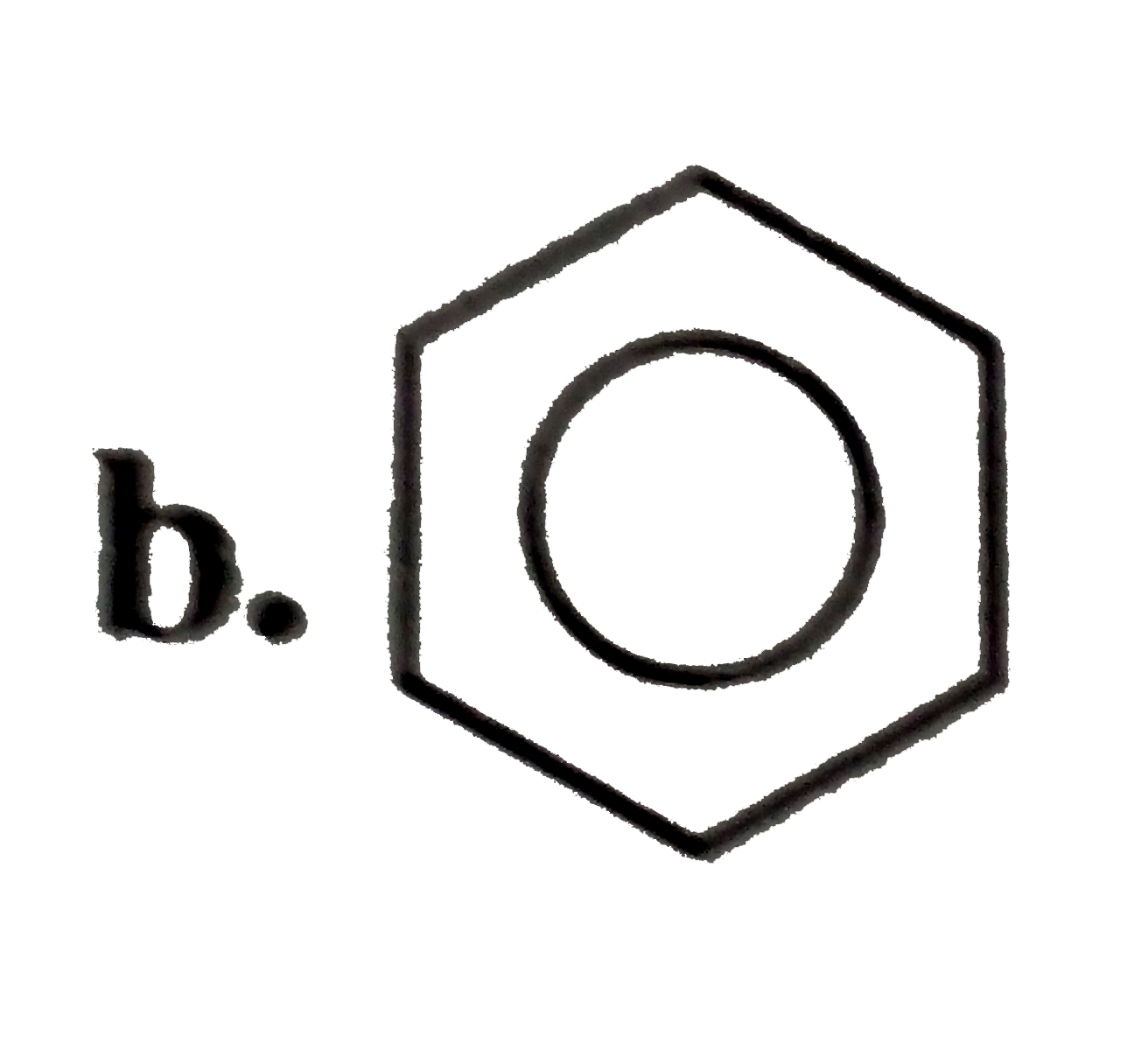
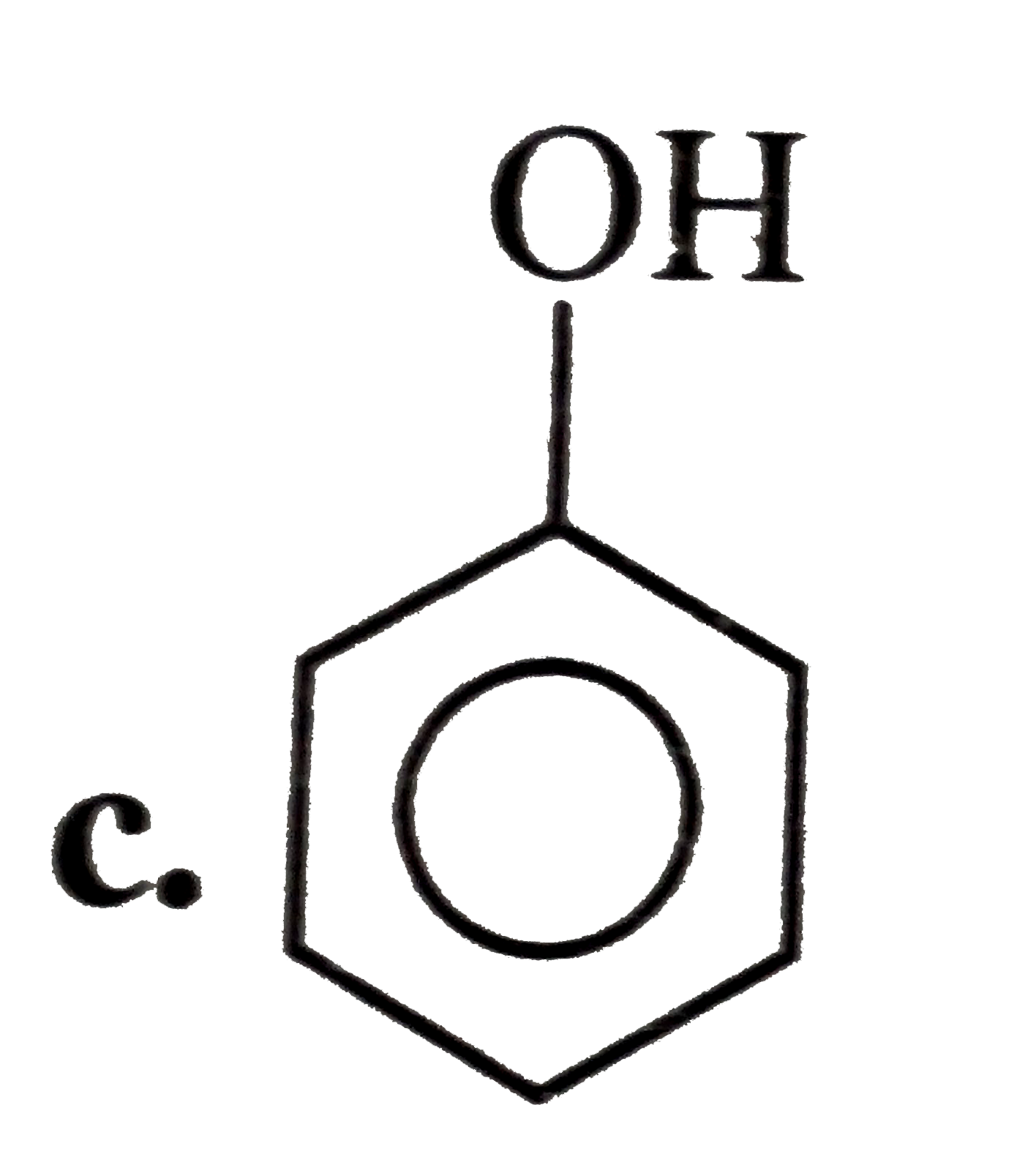
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion And Reasoning|9 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Single Correct|10 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|18 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|13 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP-Exercises Single Correct
- Which of the following is formed when RNH2 reacts with RCHO?
Text Solution
|
- Which of the following represents the poinsonous gas which caused the ...
Text Solution
|
- The conjugate base of (CH3) NH2^(o+) is :
Text Solution
|
- Which of the following is the weakest base ?
Text Solution
|
- Which of the following reaction does not yield amine?
Text Solution
|
- Primary and secondary amines are distinguished by :
Text Solution
|
- Indicate which nitrogen compound amongst the following would undergo H...
Text Solution
|
- Pick up the correct stament :
Text Solution
|
- The producet of the reaction of alcoholic silver nitrite with ethyl br...
Text Solution
|
- The electrolytic reduction of nitrobenzene in strongly acidic medium p...
Text Solution
|
- Azoxybenzene can be obtained by the treatment of mitro-benzene with :
Text Solution
|
- Tertiary nitro compounds cannot show tautomerism becauses :
Text Solution
|
- Diazo coupling is useful to prepare some
Text Solution
|
- The following reaction constituts : RNH2 + S=C=S overset (HgCl2) (r...
Text Solution
|
- Primary , secondary , tertiary amines can be separated by the followin...
Text Solution
|
- When C6 H5N2Cl is reduced with Na 2 SnOO2, the product is :
Text Solution
|
- Nitrogen is likely to be evolved when NaNO2 in dilute HCl warmed with...
Text Solution
|
- A compound X has the molecular formula C(7)H(7)NO. On treatment with B...
Text Solution
|
- A compound (X) has the molecular formula C3 H7 NO . With Br2 and KO...
Text Solution
|
- An amine on treatment with HNO2 evolved N2 The amine on exhaustive me...
Text Solution
|