Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Fill In The Blanks|3 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|13 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP-Archives Analytical And Descriptive
- Complete the following with appropriate structures : .
Text Solution
|
- Give a chemical test and the reagents used to distinguish between the ...
Text Solution
|
- Arrange the following in increasing orde of base strength : methylamin...
Text Solution
|
- How will you bring about the following conversion ? 4-nitroaniline ...
Text Solution
|
- Write the structure of the major organci product expected from the fol...
Text Solution
|
- A basic volatile nitrogen compound give a foul smelling gas when treat...
Text Solution
|
- Outline a synthesis of p-bromonitrobenzene from benzene in two steps .
Text Solution
|
- Give the structure of (A) (explanations are not required). A (C3H9N) r...
Text Solution
|
- Complete the following with appropriate structures : 2,3 "-Dinitroan...
Text Solution
|
- Write the structure of the foul-smelling compound obtained when anilin...
Text Solution
|
- Give reason for the following in one or two sectences : 'Dimethyl a...
Text Solution
|
- Following reaction gives two products . Write the structures of the pr...
Text Solution
|
- How would you bring about the following conversion (in three steps ) ?...
Text Solution
|
- There is a solution of p-hydroxybenzoic acid and p-amino benzoic acid ...
Text Solution
|
- Which of the following is more acidic and why ?
Text Solution
|
- Convert in not more than four steps. Also mention the temperature a...
Text Solution
|
- underset ((X)) underset ("Optically active") (C5 H(13)N) underset (N2)...
Text Solution
|
- A mixture of two aromatic compounds (A) and (B) separated by dissolvin...
Text Solution
|
- Convert C6H6 into PhNH2
Text Solution
|
- During acylation of amines, pyridine is added. True/False
Text Solution
|
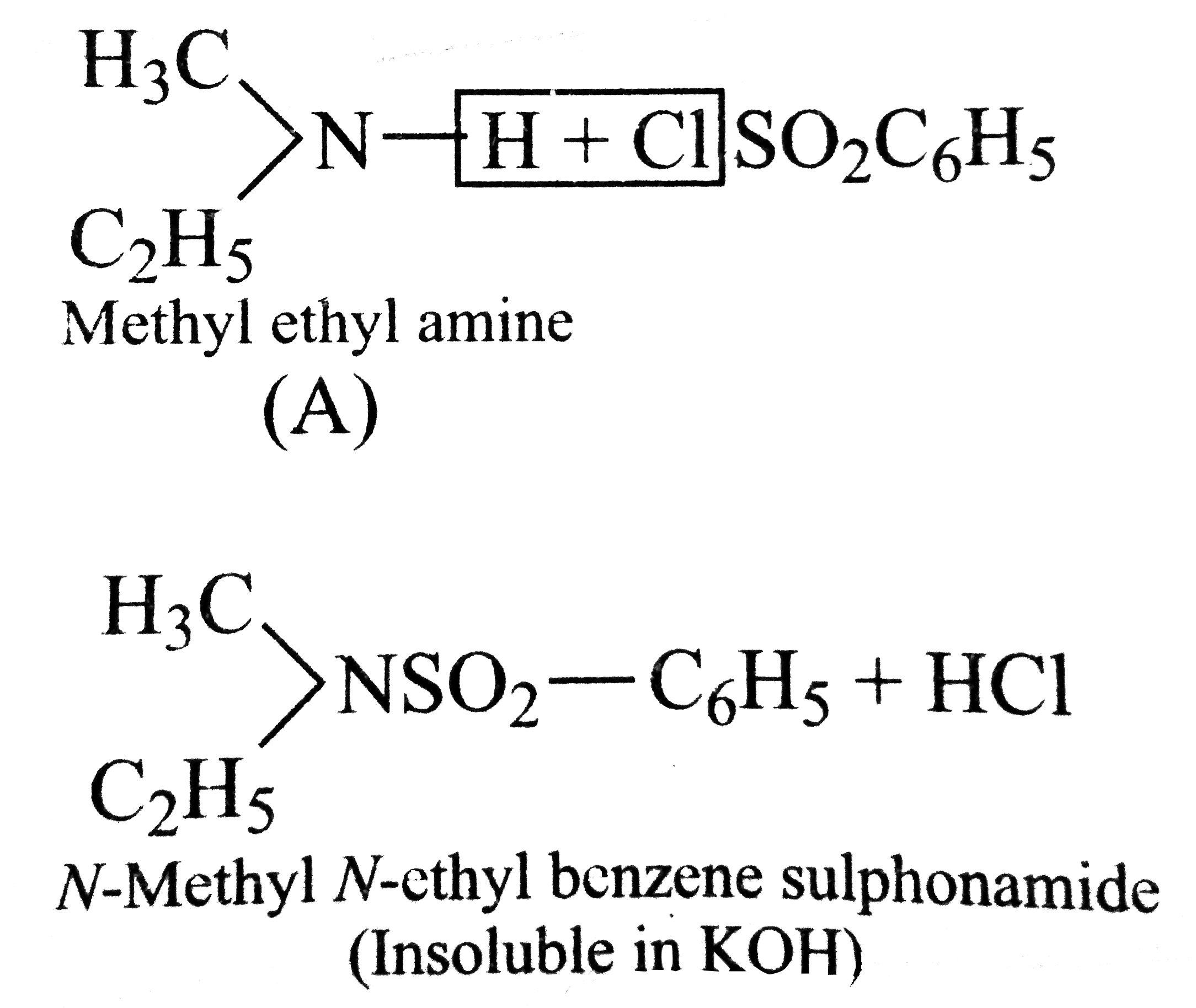 .
.