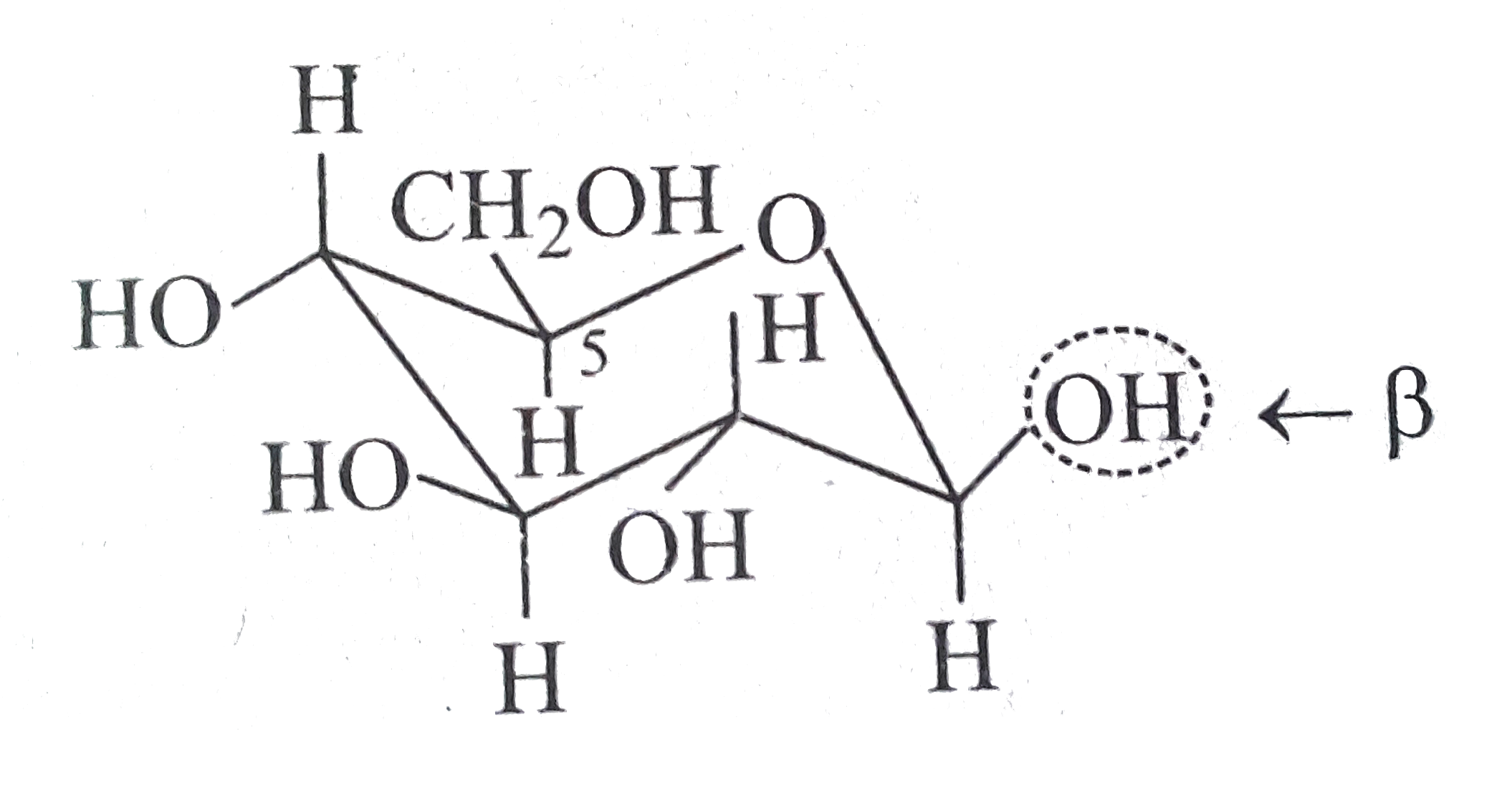
(a). Why is `beta-D-`gllucopyranose the most abundant of naturally occurring aldohexoses?
(b). Write the most stable chair conformer for `alpha-D-` fructopyranose. How does it dirrer from `beta-`anomer?
(a). Why is `beta-D-`gllucopyranose the most abundant of naturally occurring aldohexoses?
(b). Write the most stable chair conformer for `alpha-D-` fructopyranose. How does it dirrer from `beta-`anomer?
(b). Write the most stable chair conformer for `alpha-D-` fructopyranose. How does it dirrer from `beta-`anomer?
Text Solution
Verified by Experts
All the ring substituents in the chair conformation are equatorial.

Topper's Solved these Questions
BIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|45 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Concept Application)|25 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective Type|4 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 Videos
Similar Questions
Explore conceptually related problems
Statement I: beta-D- Glucophyranose is the most abundant naturally occuring aldohexoses. Statement II: All the ring substituents in the chair conformation are equatorial.
Assertion: All naturally occuring alpha -amino acids are optically active Reason: Most naturally occuring amino acids have D-configuration.
(a). Mention the most abundant and least abudant alkaline earth metal in the earth's crust. b. Arrange alkaline earth metals in order of decreasing hydration enthalpy. c. Ca, Sr and Ba generally form ionic compounds. why? d. Mention colours of Ca,Ba and Sr in flame test.
Avogadro's Number: 6.022xx10^(23) An atom of ..^(238)U disontegates by a series of alpha -decays and beta -decays until it becomes .^(206)Pb , which is stable. (i) How many alpha -decays and many beta -decays does an atom starting as ..^(238)U undergo before it becomes stable? (ii) One of the following ten nuclides is formed from a series of disintegrations starting at ..^(238)U . Which one? .^(235)U, .^(234)U, .^(228)Ac, .^(224)Ra, .^(224)Rn, .^(220)Rn, .^(215)Po, .^(212)Po, .^(212)Pb, .^(211)Pb .
The IUPAC definition of a transition element is that it is an element that has an incomplete d-subshell in either the neutral atom or its ion. Thus the group 12 elements are member of the d-block but are not transition elements. Chemically solft members of the d-block occurs as sulphide minerals and are partially oxidised to obtain the metal, the more electropositive 'hard' metals occurs as oxides and are extracted by reduction. Opposite to p-block elements, the higher oxidation states are favoured by the heavier elements of d-block Metals on the right of the d-block tend to exist in low oxidation states and form complexes with the ligands. Square-planar complexes are common for the platinum metals and gold in oxidation states that yield d^8 electronic configuration, which include RH(I),Ir(I),Pd(II),Pt(II) and Au(III). The most distinctive features/properties of transition metal complex is their wide range of colours.The crystal field theory attributes the colour of the coordination compounds to d-d transition of the electron.It is important to note that (a) in absence of ligand, crystal field spilling does not occur and hence the substances is colourless, (b) the type of ligand also influences the colour of the complexes. Which of the following has dsp^2 hybridisation and is diamagnetic in nature ? (i) Na_4[Cr(CO)_4] , (ii) [Ni(DMGH)_2] , (iii) [PtHBr(PEt_3)_2] (iv) [As(SCN)_4]^(3-) , (v) [AuBr_4]^(-)
The diagram given below is a representation of a phenomenon pertaining to the nervous system. Study the diagram and answer the following questions: (i) Name the phenomenon that is being depicted. (ii) Give the technical term for the point of contact between the two nerve cells. (iii) Name the parts A, B, C and D. (iv) Write the functions of parts E and F. (v) How does the arrangement of neurons in the spinal cord differ from that of the brain.
Hydrogen accounts for approximately 75% of the mass of the universe. Hydrogen serves as the nuclear fuel of our Sun and other stars, and these are mainly composed of hydrogen. On the earth, though hydrogen is rarely found in the uncombined state. Since the earth's gravity is too weak to hold such light molecules, nearly all the H_2 originally present in the earth's atmosphere has been lost to space. In the earth's crust and oceans, hydrogen is found in water, petroleum, proteins, carbohydrates and other compounds and it is the ninth most abundant element on a mass basis. Hydrogen has three isotopes : hydrogen or protium () , deuterium or heavy hydrogen (D or ), tritium (T or ) . The physical properties of the three isotopes are different due to the difference in their masses, i.e. isotope effect. The chemical properties of the three isotopes are similar as they have the same electronic configuration. Reaction between hydrogen and oxygen is highly exothermic, and gas mixtures that contain as little as 4% by volume hydrogen in oxygen (or in air) are highly flammable and potentially explosive. 2H_(2(g))+O_(2(g)), DeltaH^(ɵ)=-485 kJmol^(-1) As hydrogen is environmentally clean it is an enormously attractive fuel. 'Hydrogen economy' is an emerging field in which it is thought that our energy needs can be met by gaseous, liquid and solid hydrogen. As hydrogen is no a naturally occuring substance such as coal, oil or natural gas, energy must be exploaded to produce hydrogen before it can be used. Hydrogen, H_2 is very less abundant in the atmosphere due to
Hydrogen accounts for approximately 75% of the mass of the universe. Hydrogen serves as the nuclear fuel of our Sun and other stars, and these are mainly composed of hydrogen. On the earth, though hydrogen is rarely found in the uncombined state. Since the earth's gravity is too weak to hold such light molecules, nearly all the H_2 originally present in the earth's atmosphere has been lost to space. In the earth's crust and oceans, hydrogen is found in water, petroleum, proteins, carbohydrates and other compounds and it is the ninth most abundant element on a mass basis. Hydrogen has three isotopes : hydrogen or protium () , deuterium or heavy hydrogen (D or ), tritium (T or ) . The physical properties of the three isotopes are different due to the difference in their masses, i.e. isotope effect. The chemical properties of the three isotopes are similar as they have the same electronic configuration. Reaction between hydrogen and oxygen is highly exothermic, and gas mixtures that contain as little as 4% by volume hydrogen in oxygen (or in air) are highly flammable and potentially explosive. 2H_(2(g))+O_(2(g)), DeltaH^(ɵ)=-485 kJmol^(-1) As hydrogen is environmentally clean it is an enormously attractive fuel. 'Hydrogen economy' is an emerging field in which it is thought that our energy needs can be met by gaseous, liquid and solid hydrogen. As hydrogen is no a naturally occuring substance such as coal, oil or natural gas, energy must be exploaded to produce hydrogen before it can be used. Which of the following is radioactive in nature?
Hydrogen accounts for approximately 75% of the mass of the universe. Hydrogen serves as the nuclear fuel of our Sun and other stars, and these are mainly composed of hydrogen. On the earth, though hydrogen is rarely found in the uncombined state. Since the earth's gravity is too weak to hold such light molecules, nearly all the H_2 originally present in the earth's atmosphere has been lost to space. In the earth's crust and oceans, hydrogen is found in water, petroleum, proteins, carbohydrates and other compounds and it is the ninth most abundant element on a mass basis. Hydrogen has three isotopes : hydrogen or protium (H) , deuterium or heavy hydrogen (D or ), tritium (T or ) . The physical properties of the three isotopes are different due to the difference in their masses, i.e. isotope effect. The chemical properties of the three isotopes are similar as they have the same electronic configuration. Reaction between hydrogen and oxygen is highly exothermic, and gas mixtures that contain as little as 4% by volume hydrogen in oxygen (or in air) are highly flammable and potentially explosive. 2H_(2(g))+O_(2(g)), DeltaH^(ɵ)=-485 kJmol^(-1) As hydrogen is environmentally clean it is an enormously attractive fuel. 'Hydrogen economy' is an emerging field in which it is thought that our energy needs can be met by gaseous, liquid and solid hydrogen. As hydrogen is no a naturally occuring substance such as coal, oil or natural gas, energy must be exploaded to produce hydrogen before it can be used. If an isotope of hydrogen has one neutron in its atom, its atomic number and atomic mass will respectively be
Hydrogen accounts for approximately 75% of the mass of the universe. Hydrogen serves as the nuclear fuel of our Sun and other stars, and these are mainly composed of hydrogen. On the earth, though hydrogen is rarely found in the uncombined state. Since the earth's gravity is too weak to hold such light molecules, nearly all the H_2 originally present in the earth's atmosphere has been lost to space. In the earth's crust and oceans, hydrogen is found in water, petroleum, proteins, carbohydrates and other compounds and it is the ninth most abundant element on a mass basis. Hydrogen has three isotopes : hydrogen or protium () , deuterium or heavy hydrogen (D or ), tritium (T or ) . The physical properties of the three isotopes are different due to the difference in their masses, i.e. isotope effect. The chemical properties of the three isotopes are similar as they have the same electronic configuration. Reaction between hydrogen and oxygen is highly exothermic, and gas mixtures that contain as little as 4% by volume hydrogen in oxygen (or in air) are highly flammable and potentially explosive. 2H_(2(g))+O_(2(g)), DeltaH^(ɵ)=-485 kJmol^(-1) As hydrogen is environmentally clean it is an enormously attractive fuel. 'Hydrogen economy' is an emerging field in which it is thought that our energy needs can be met by gaseous, liquid and solid hydrogen. As hydrogen is no a naturally occuring substance such as coal, oil or natural gas, energy must be exploaded to produce hydrogen before it can be used. Which of the following fuel produces least environmental pollution?
CENGAGE CHEMISTRY ENGLISH-BIOMOLECULES-Exercises Archives (Analytical And Descriptive)
- (a). Why is beta-D-gllucopyranose the most abundant of naturally occur...
Text Solution
|
- Alanine, the amino acid the respective structure at pH = 2 and pH = 10...
Text Solution
|
- Give the structure of each of the products in the following reactions:...
Text Solution
|
- As partune, an artifical sweetener, is a peptide and has the following...
Text Solution
|
- Write down the heteroheneous catalyst involved in the polymerisation o...
Text Solution
|
- Following two amino acids liosine and glutamine form dipeptide linkage...
Text Solution
|
- (a) Draw the structure of L-glucose. (b) Give the reaction of L-gluc...
Text Solution
|
- Which of the following disaccharides will not reduce Tollens reagent?
Text Solution
|
- Arrange in the order of increasing acidic strengths.
Text Solution
|