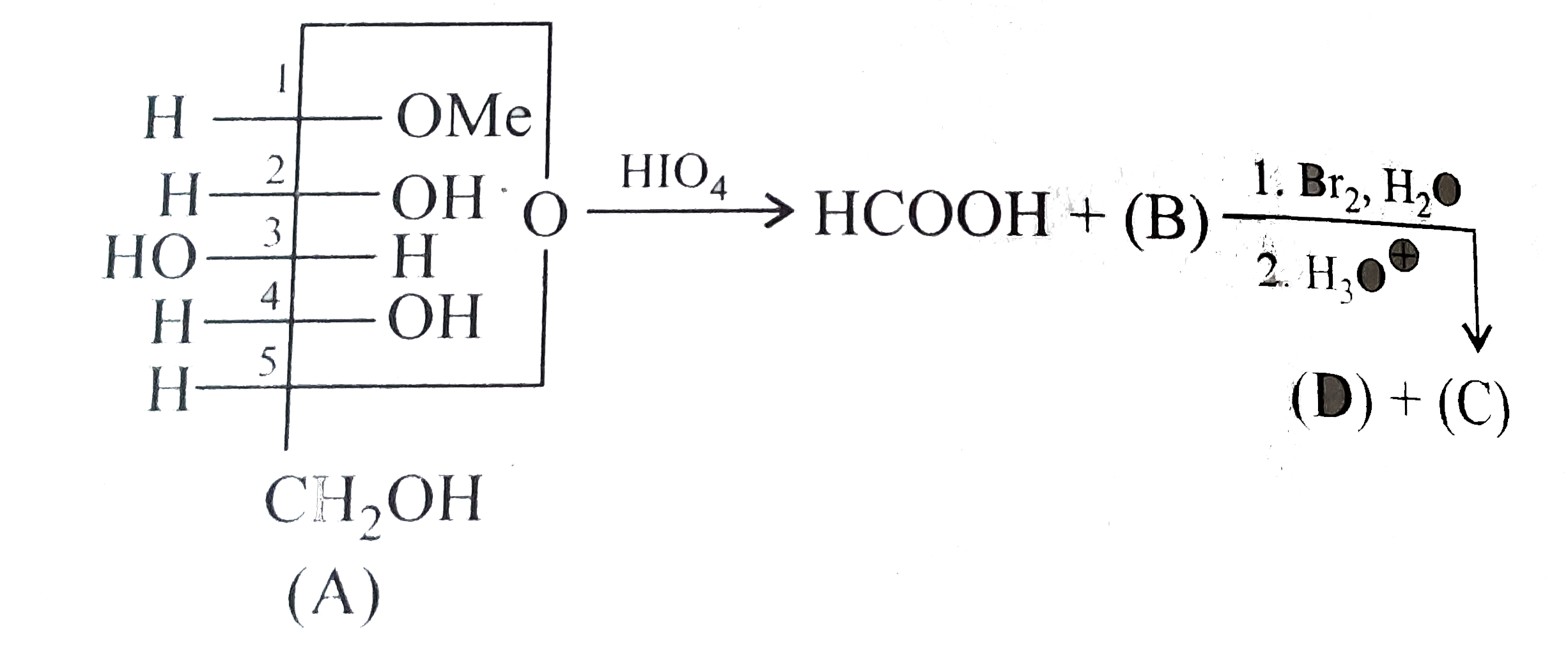
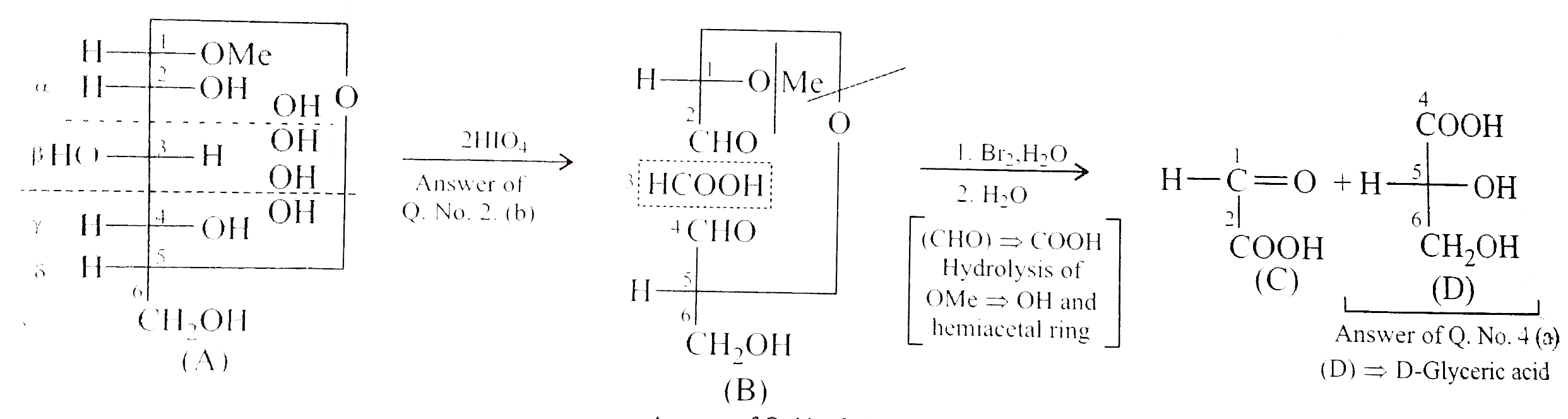
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correcttype)|30 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correcttype)|80 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Concept Application)|25 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective Type|4 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-BIOMOLECULES-Exercises (Linked Comprehension)
- The name of compound (A) is:
Text Solution
|
- The number of moles of HIO(4) required in the above reaction is:
Text Solution
|
- The structure of (B) is:
Text Solution
|
- Compound (C ) and (D) are:
Text Solution
|
- The name of compound (A) is:
Text Solution
|
- The number of moles of HIO(4) required in the above reaction is:
Text Solution
|
- The structure of (B) is?
Text Solution
|
- Compound (C ) and (D) are:
Text Solution
|
- Which sta tement(S) is//are correct about (A)?
Text Solution
|
- Compound (B) is:
Text Solution
|
- Compound (C ) is:
Text Solution
|
- Compound (D) is:
Text Solution
|
- Which statement(S) is//are correct about the products (E) and (F)?
Text Solution
|
- Which statement(S) is//are correct about (A)?
Text Solution
|
- Compound (B) is:
Text Solution
|
- Compound (C ) is:
Text Solution
|
- Compound (D) is:
Text Solution
|
- Which statemetn(S) is//are correct about the products from (D) by path...
Text Solution
|
- Compound (B) is:
Text Solution
|
- How many moles of PhNHNH(2) react with 1 mol (A)?
Text Solution
|