Text Solution
Verified by Experts
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise EXERCISES|29 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise linked Comprehension Type|38 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct Answer|14 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
CENGAGE CHEMISTRY ENGLISH-ALKENES AND ALKADIENES-SOLVED Example
- Write the monomer of the following polymer : a. P.M.M.A. ,b. H.D.P....
Text Solution
|
- Give examples of some optically active polymers
Text Solution
|
- Based on the arrangement of branching of [(R)-(Ph), in case of styron ...
Text Solution
|
- Convert the following :
Text Solution
|
- Identify compound (A).
Text Solution
|
- Identify compound (A) and (B).
Text Solution
|
- Identify compounds (A) and (B)
Text Solution
|
- Write the products of the reductive and oxidative ozonolyses of mesity...
Text Solution
|
- Identify compound (A) in the following reactions. a.Compound (A)O(3)...
Text Solution
|
- Give the product of the following with : a. O(3)//Zn-CH(3)COOH ,b. ...
Text Solution
|
- Write the structural formulae for the compounds that yields the follow...
Text Solution
|
- Give the product obtained from the oxidation of the following compound...
Text Solution
|
- Find C
Text Solution
|
- Comlete the following reaction : Also write the mechanism of th...
Text Solution
|
- Give all the structural isomers ( excloding stereoisomers and allenes ...
Text Solution
|
- Complete the following reaction : Also write the mechanism of the...
Text Solution
|
- Write all the resonating structures of : Which of these carbons ...
Text Solution
|
- Convert :
Text Solution
|
- Give the name and structure of the lowest molecular mass cumulative po...
Text Solution
|
- Complete the reaction
Text Solution
|
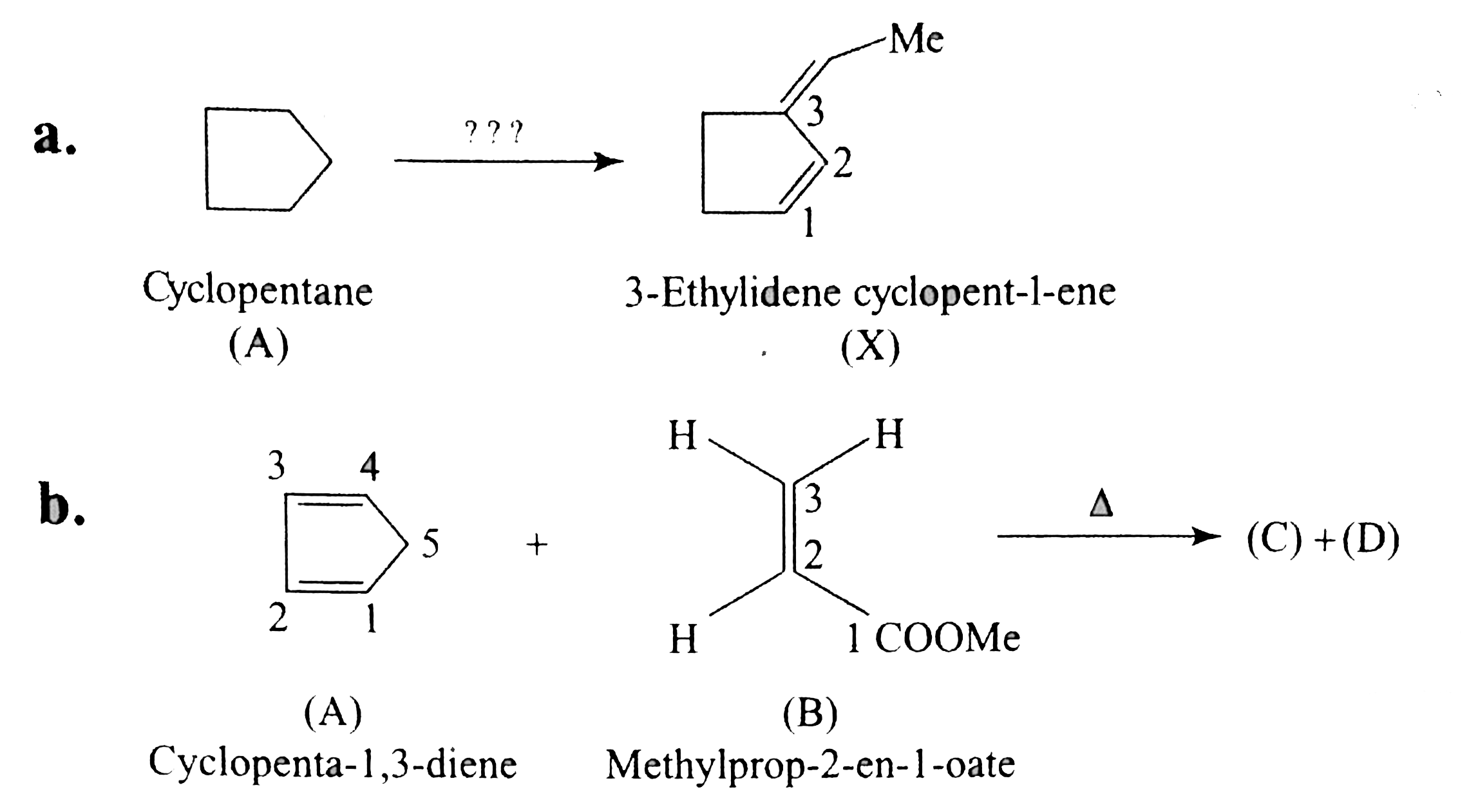
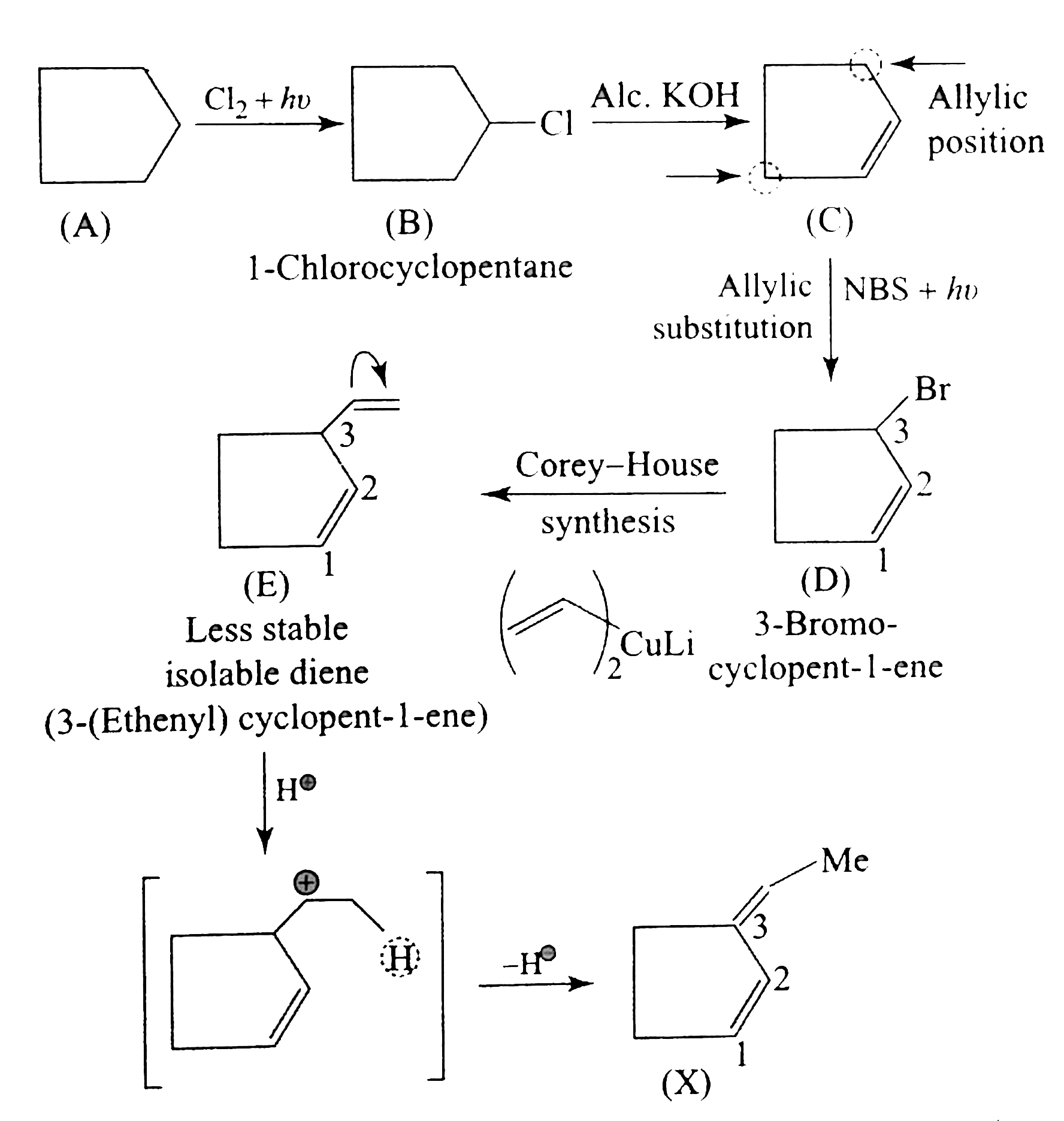
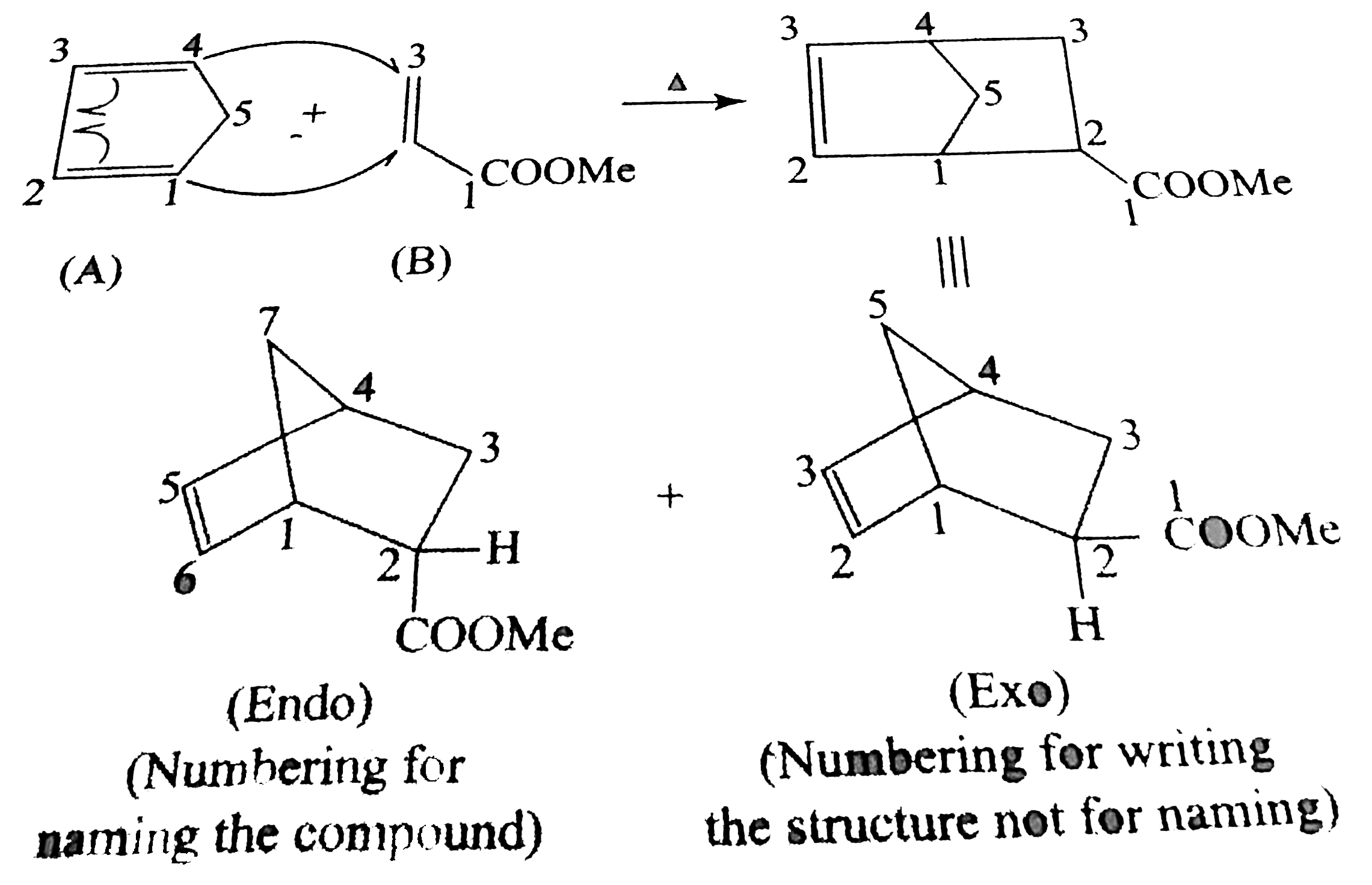
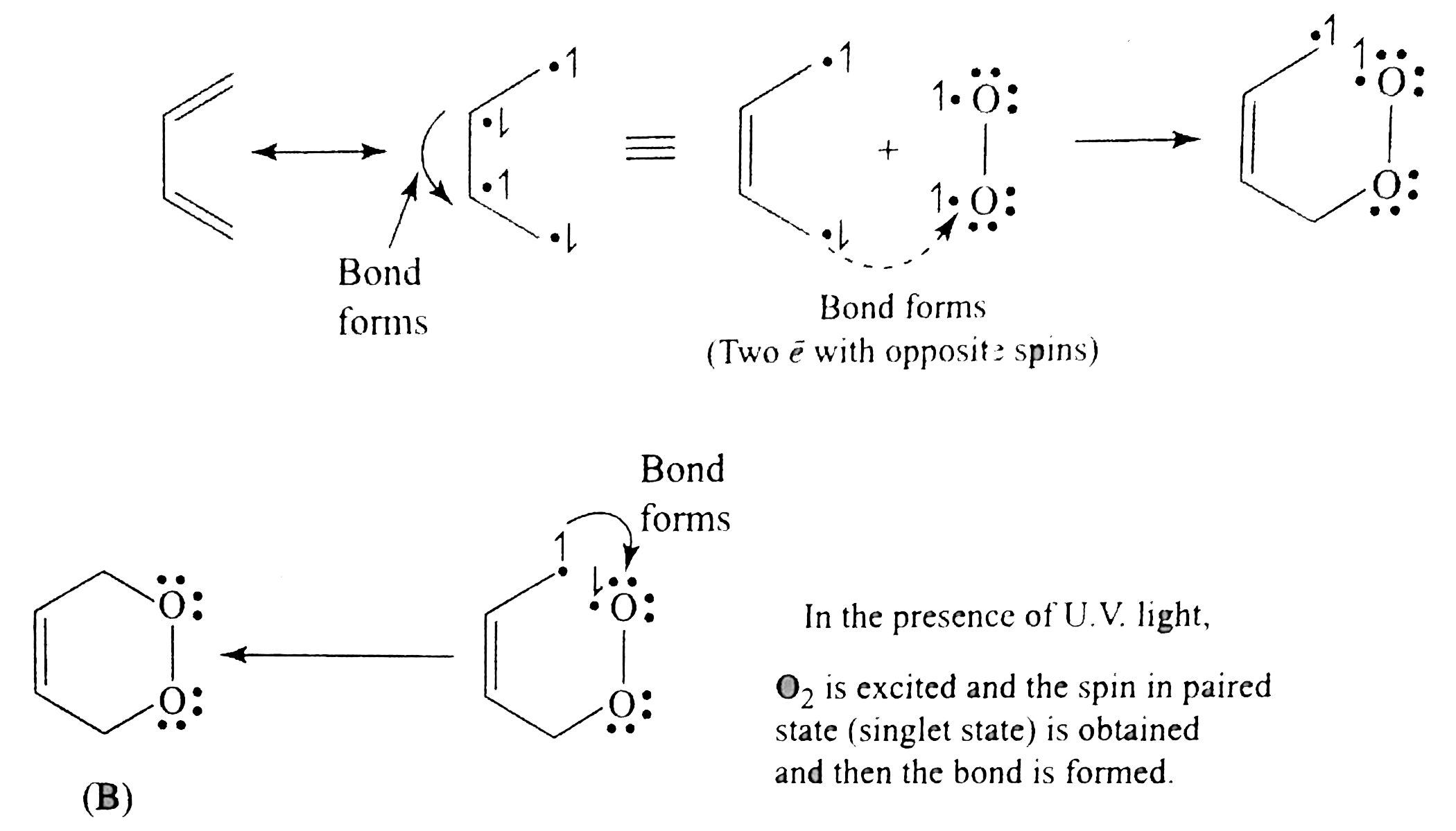
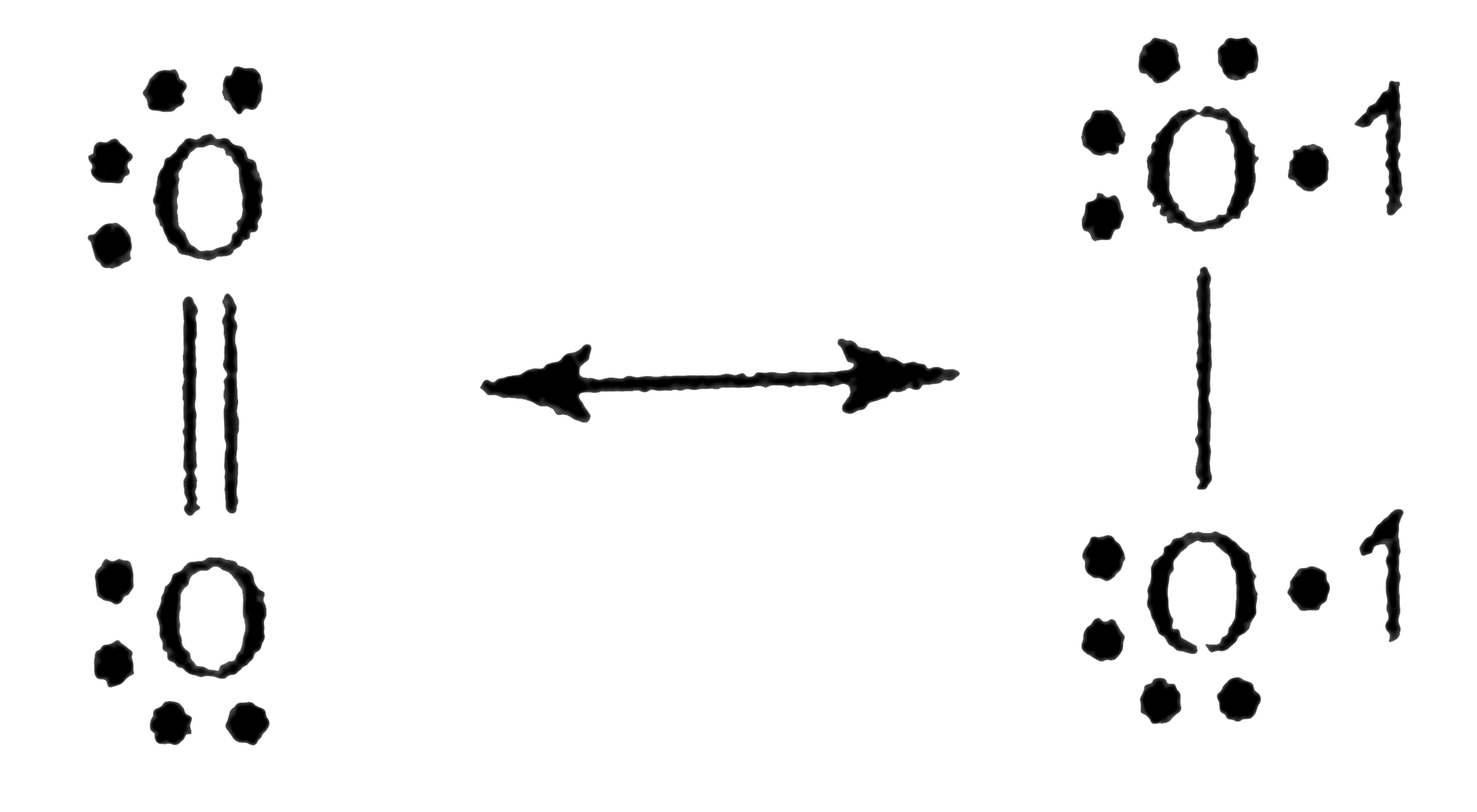 `O_(2)` is a diradical with the same spin, so it can form one bond with diene, forming an intermediat and does not form the second bond because it has two electrons with the same spins (triplet state)
`O_(2)` is a diradical with the same spin, so it can form one bond with diene, forming an intermediat and does not form the second bond because it has two electrons with the same spins (triplet state)